The prevalence of diabetes continues to increase globally, and the International Diabetes Foundation (2021) estimated that 537 million adults aged 20 to 79 worldwide were living with diabetes in 2021. This situation leads to an increasing incidence of complications of the foot, including infections. Diabetes-related foot infections (DFIs) are associated with substantial morbidity and require frequent medical care, such as doctor visits, daily wound care, antimicrobial therapy, surgical procedures and high healthcare costs (Raspovic and Wukich, 2014).
Of particular importance, DFIs remain the most frequent diabetes-related complications requiring hospitalisation and constitute the most common triggering cause leading to lower limb amputation (Peters et al, 2001; Lavery et al, 2007). Outcomes in patients presenting with an infected diabetes-related foot ulcer (DFU) are suboptimal; in a large prospective study, at the end of 1 year, the ulcer had healed in only 46% (and subsequently recurred in 10% of these), while 15% had died and 17% had required lower limb amputation (Ndosi et al, 2018). Given these data, it is essential to be able to heal DFUs in a short time in order to avoid serious infectious complications that can put the patient’s health at risk. The authors of this case report believe that highlighting and sharing the results of this study can contribute to improvements in the local management of DFUs.
Case report
A 71-year-old man presented to the emergency room for a reported episode of disorientation and tremors with subsequent stiffening of the lower limbs and subsequent fall from bed. A brain CT scan was performed, which excluded ongoing acute alterations. His relevant medical history included: arterial hypertension, type 2 diabetes, arterial occlusive critical pathology of the lower limbs, heart disease, chronic ischaemia with a pacemaker, atrial fibrillation on direct oral anticoagulants, chronic kidney disease (class IIIa), psoriasis, numerous hospitalisations for infections, glyco-metabolic decompensation and ischaemic stroke.
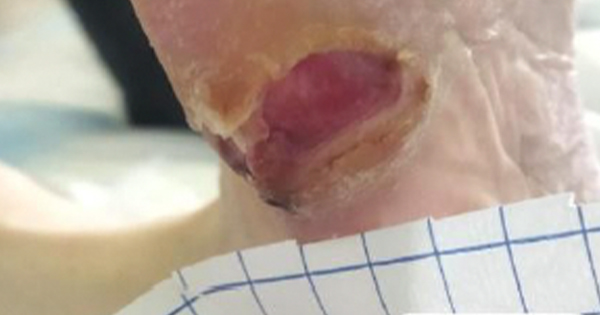
Upon admission to the geriatrics department, examination showed a neuropathic ulcer (SINBAD score 4) measuring 3 cm × 2 cm with a depth of 0.5 cm at the base of the fifth radius of the right foot. This had been treated on an outpatient basis for approximately 6 weeks with a hydrofiber and silver dressing (Aquacel AG+ Extra; Convatec). The ulcer had a dry bottom, purulent exudate, inactive edges, hyperkeratotic perilesional skin and was causing severe pain. Blood tests showed no neutrophilic leukocytosis, CRP (5.6), hyperglycaemia (143 mg/dl), HbA1c 87 mmol/mol and a slight reduction in renal function.
A deep swab of the wound was performed with the Levine technique and this tested positive for Proteus mirabilis sensitive to the established therapy (piperacillin/tazobactam). Blood cultures were negative. Debridement of the hyperkeratotic tissue and deep cleaning of the wound were performed according to the principles of wound bed preparation with saline solution and antisepsis via compresses with 0.005% sodium hypochlorite. A bacterial capture dressing coated with dialkylcarbamoylchloride (DACC) and impregnated with hydrogel (Cutimed Sorbact Gel, Essity) was used and changed every 48–72 hours [Figure 1].
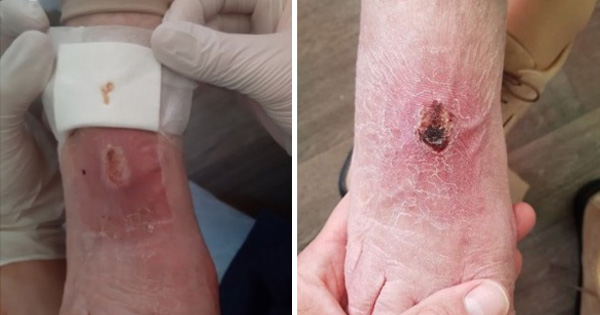
After the first change, the lesion appeared superficial with a bright red granulation tissue and without the presence of purulent exudate, the pain has significantly reduced. At subsequent changes fibrin and new perilesional hyperkeratotic tissue was observed; this was due to the fact that the patient had delirium with wandering, especially nocturnal, and he walked frequently without adequate relief footwear at these times. Frequent debridement and the reduction in the frequency of dressing changes did see a progressive reduction of the ulcer up to substantial epithelialization and closure after 10 days of hospitalisation [Figure 2].
During hospitalisation, the patient underwent a foot X-ray that excluded osteomyelitic lesions, Doppler ultrasound with the presence of direct flow to the tibials and feet, and diabetes consultations to remodulate his insulin therapy, given the high HbA1c.
Discussion
DFUs affect approximately 20 million people worldwide each year and without proper treatment, they can lead to infection, hospitalisation, amputation and death (Boulton et al, 2005; Armstrong et al, 2017; Zhang et al, 2020; Lazzarini et al, 2023). Therefore, healing of DFUs is of critical global importance. The most common cause of DFU is the high mechanical stress of the foot tissues related to a reduced perception of pain, which means that repeated trauma can reach the point of causing an injury (Fernando et al, 2014; Bus, 2016; Armstrong et al, 2017). The cause of this phenomenon can be attributed to sensory neuropathy which affects approximately half of all people with diabetes (Armstrong et al, 2017; Jeffcoate et al, 2018; Lazzarini et al, 2018).
Mechanical tissue stress is composed of plantar pressures and shear forces accumulated during repetitive cycles of weight-bearing activities (Fernando et al, 2014; Bus, 2016; Armstrong et al, 2017). Peripheral neuropathy can also lead to further changes in gait, foot deformity and soft tissue which can further increase mechanical stress on the tissues (Fernando et al, 2013, 2014; Lazzarini et al, 2019). Once a DFU forms, healing is delayed if the area is not effectively offloaded and if proper wound management is not performed to exclude the presence of arterial insufficiency (International Working Group on the Diabetic Foot [IWGDF], 2023).
In wound management, a key role, in addition to wound bed preparation, is played by the choice of dressing. In this clinical case we saw how the bacterial capture dressing coated with DACC and impregnated with hydrogel significantly contributed to closing the wound by managing infection and pain from the first changes.
The effective infection management of this dressing was demonstrated in a recent systematic review which extended the evidence for the antimicrobial effect of DACC-coated dressings, particularly regarding the antimicrobial effect against priority microorganisms reported by World Health Organization (Rippon et al 2021). The dressing, applied directly to the wound bed, captures bacteria and fungi thanks to the hydrophobic interaction. Pathogens are irreversibly bound and removed with each dressing change without releasing active ingredients and it is for this reason that it is a valid ally in the fight against antimicrobial resistance (Rippon et al 2021, 2023). The hyperosmolarity of the hydrogel makes instead, the disintegration of fibrin, slough and biofilm is possible as well as promoting a moist and optimal environment for wound healing (IWGDF, 2023). The ability to promote granulation and wound closure, as happened in the clinical case, was recently demonstrated by an interesting clinical study which analysed the dressing in vitro and observed how DACC influences it by inducing the expression of growth factors resulting in potential positive effects on fibroblasts and keratinocytes (Morgner et al, 2022; Rippon et al, 2023).
Conclusion
Foot ulceration is a major complication of diabetes and is associated with high levels of morbidity and mortality, as well as significant financial costs. Early closure of DFUs is essential to prevent the onset of serious infectious complications that can put the health of our patients at risk. In this clinical case, the bacterial capture dressing coated with DACC and impregnated with hydrogel proved to be optimal in managing both the infectious component and wound healing in a significantly short time. It is hoped that in the future this product will be included among the gold standards in the treatment of DFUs; to this end, further multicentre clinics and systematic reviews that include greater sampling are needed.