Calcinosis cutis is a rare disorder first described by Virchow in 1855 characterized by abnormal deposition of amorphous calcium salts under the epidermis in various parts of the body. Scleroderma is a connective tissue disorder with a complex and largely unclear pathogenesis (Mourad and Louis, 2021).
Case report
A 54-year-old female presented to our wound clinic on 4 May 2023 complaining of an ulcer on the left anterior mid-tibia. She stated that the ulcer had been present for about 2 months. She had previously been seen in urgent care on two previous occasions. Initially, she was presumptively placed on sulphamethoxazole/trimethoprim double strength for 10 days, and a wound culture was performed. Two weeks later, she returned to convenient care and was placed on clindamycin after the culture was positive for Staphylococcus aureus. After her second urgent care visit, she was referred to us for surgical debridement.
She had previously been seen in the emergency department in November 2017 for leg pain. A venous ultrasound was negative for deep vein thrombosis, and an x-ray of the tibia revealed some soft tissue calcifications reported as consistent with her history of scleroderma. At that time, there was no ulcer. A photograph of her left calf taken by primary care at her visit for leg pain in July 2021 also showed no ulcer. She had a parathyroid hormone level in 2014, which was 155 pg/mL and again in 2016, which was 177 pg/mL (15–65 pg/mlL normal range).
Her past medical history included depression, migraine headaches, scleroderma (HCC), and psoriasis. Her past surgical history included gastric bypass surgery and repair of an umbilical hernia. She had not seen her rheumatologist in 4 years and was not prescribed any rheumatologic medications.
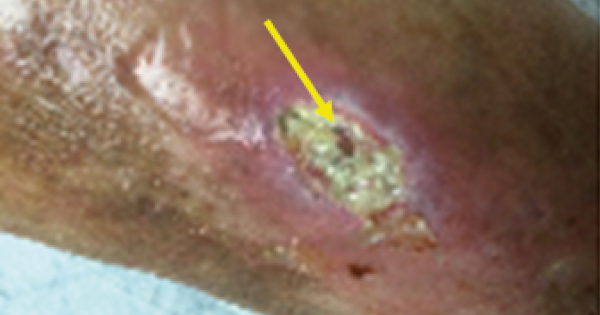
Upon presentation to the wound clinic, physical examination revealed a 2.5 × 1.5 × 0.1 cm ulcer mid anterior left tibia. Figure 1 shows a calcific deposit that was removed during surgical debridement. There were several other calcific densities that were also debrided at the time.
Calcinosis
The calcinosis that is present in scleroderma may result from an active process involving dysregulated mesenchymal stem cell differentiation, leading to a bone-forming microenvironment. Calcinosis cutis (CC) is the deposition of calcium in the skin and subcutaneous tissues, often associated with debilitating complications, such as pain, recurrent episodes of local inflammation, and functional impairment (Valenzuela and Chung, 2015). Traditionally, three types of calcinosis cutis have been distinguished: metastatic, dystrophic, and idiopathic. Some add a fourth type called iatrogenic (Walsh and Fairley, 1995; Moss et al, 2006). Dystrophic calcinosis usually occurs in the dermis, which has previously been damaged. In the pathophysiology of the disease, damage to the elastic fibers has been proposed (Cochran and Wilkin, 1938). The deposit can be localized, for example, in cutaneous tumors, cysts, local traumas (Sell et al, 1980; Ellis et al, 1984; Pitt et al, 1990; Lee et al, 2005), burns (Coskey and Mehregan, 1984), or frostbite (Larson et al, 1984). Occasionally, percutaneous penetration has been suggested as the pathogenic mechanism (Wheeland and Roundtree, 1985). On the contrary, it can also be widespread in conditions, such as dermatomyositis (Gushi, 2002; Jat and Singh, 2008; Lobo et al, 2008; Wu and Metz, 2008), scleroderma (Ellis et al, 1984; Vereecken et al, 1998), lupus erythematosus (Simons-Ling et al, 1983; Johansson et al, 1988, Rothe et al, 1990; Yamamoto et al, 2007), pseudoxanthoma elasticum (Reeve et al, 1979, Nikko et al, 1996, Buka et al, 2000; Federico et al, 2008), or in Ehlers-Danlos syndrome (Black and Kanat, 1985), just to mention some examples. Herpes infection is a rare cause of this widespread type of calcinosis (Beers et al, 1986). In some publications, when dystrophic calcification is widespread, it is catalogued as “calcinosis universalis” (Walsh and Fairley, 1995), a term that some prefer to use only for widespread idiopathic calcinosis (McKee et al, 2005). According to the literature, nearly 25% to 40% of patients with limited cutaneous systemic sclerosis will develop calcinosis within 10 years of disease onset (Balin et al, 2012).
Pathology
Calcium hydroxyapatite has been identified as the major component of soft tissue calcinosis in scleroderma patients (Hsu, et al, 2017). The composition of the calcinosis is that it resembles that of bone and not of enamel (Hsu, et al, 2017). The formation of calcified deposits is based on the initial tissue insult and serum levels of calcium and phosphorous (Mourad and Louis, 2021; Valenzuela and Chung, 2015; Walsh and Fairley, 1995; Moss et al, 2006; Cochran and Wilkin, 1938; Sell et al, 1980; Ellis et al, 1984; Pitt et al, 1990; Lee et al, 2005; Coskey and Mehregan, 1984; Larson et al, 1992; Wheeland and Roundtree, 1985; Gushi et al, 2002; Jat and Singh, 2008; Lobo et al, 2008; Wu and Metz, 2008; Vereecken et al, 1998; Simons-Ling et al, 1983; Johansson et al, 1988; Rothe et al, 1990; Yamamoto et al, 2007; Reeve et al, 1979; Nikko et al, 1996; Buka et al, 2000; Federico et al, 2008; Black and Kanat, 1985; Beers et al, 1986; McKee et al, 2005; Hsu et al, 2017; Wasserman et al, 2020; Elahmar et al, 2022; Eidelman et al, 2009; Mukamel et al, 2001). The pathophysiology of calcinosis cutis is poorly understood. The presence of calcium within the wound bed serves to upregulate the presence of inflammatory markers, leading to infection, increased wound exudate and impaired healing (Wasserman et al, 2020). The pathophysiology behind dystrophic calcinosis cutis has several proposed mechanisms: chronic inflammation, vascular hypoxia, recurrent trauma, and abnormalities in bone matrix proteins (Elahmar et al, 2022).
The role of inflammation has been established to some extent in the pathophysiology of calcinosis (Mukamel et al, 2001). Here, it has been found that IL-6, IL-2b, and tumor necrosis factor are present in the subcutaneous fluid (Osthoff et al, 2014). It has been noted in several studies that vascular hypoxia contributes to calcinosis (Davies et al, 2006). It has been observed that there is an increase in the expression of advanced glycation/lipoperoxidation end products (AGEs, which are markers of oxidative stress that can occur due to ischaemic reperfusion injury) in patients with calcinosis (Davies et al, 2006).
Association with other diseases
Hypothyroidism occurs more frequently in women with scleroderma than in control populations (Marasini et al, 2007; Antonelli et al, 2007). Fibrosis of the thyroid gland can directly cause hypothyroidism in patients with scleroderma and appears to be responsible for most cases (Antonelli et al, 2007; Gordon et al, 1981; Kahl et al, 1986). In a prospective study of 179 patients with scleroderma, baseline thyroid-stimulating hormone was higher and anti-thyroid peroxidase (anti-TPO) antibodies were more likely to be present in scleroderma patients than controls (Antonelli et al, 2013). In a study of 138 patients with scleroderma, anti-TPO antibodies were increased in patients with limited scleroderma, whereas the levels of these antibodies in patients with diffuse scleroderma were similar to those in healthy controls (Danielides et al, 2011). In an Italian series of 327 patients with systemic sclerosis (52% diffuse, i.e., scleroderma), there was an increased prevalence of anti-TPO and antithyroglobulin antibodies in comparison with controls (Antonelli et al, 2016). Autoimmune destruction of the parathyroid can lead to hypoparathyroidism (Shoback, 2008).
Diagnosis
Plain radiography has been shown to be very sensitive in detecting calcinosis (Shahi et al, 2014). In most cases, when a patient presents to a wound clinic and calcinosis cutis is found, the patient has already been diagnosed with a causative condition. If no diagnostic investigations have been undertaken, evaluation of calcium, phosphorous, thyroid and parathyroid function should be performed. Submitting debrided calcific material to pathology will also confirm the diagnosis.
Treatment
Most treatment modalities for calcinosis cutis are outside the scope of wound care practice. There is one treatment of interest (minocycline) other than standard wound care that can be instituted in the wound clinic.
Minocycline
Minocycline is a broad-spectrum antibiotic in the tetracycline family. It is recognised that tetracyclines have other actions and effects in addition to their antibacterial action. They chelate calcium and iron (Cohen et al, 1998), can influence osteoclast function (Donahue et al, 1992), inhibit collagenases, and are potent inhibitors of neutrophil matrix metalloproteinase (MMP) function (Gabler and Creamer, 1991). They have anti-inflammatory actions and suppress polymorphonuclear neutrophil activity, inhibit the cyclo-oxygenase pathway, and scavenge for reactive O2 metabolites produced by polymorphonuclear neutrophils that play a part in tissue destruction (Gabler and Creamer, 1991). Tetracyclines also directly inhibit collagenolytic enzymes, including matrix MMPs, elastase and cathepsins. These properties have been of interest in conditions that are characterised by overproduction of these enzymes, and there has been some success in treating non-infected corneal ulcers (Perry and Golub, 1985), blistering skin disorders (Berk and Lorincz, 1986), rheumatoid arthritis (O’Dell et al, 2001), and scleroderma (Le et al, 1998). One of the main actions of minocycline in patients is through its MMP inhibitory activity, thus reducing the inflammation and ulceration associated with calcinosis deposits (Robertson et al, 2003).
An early report relayed the effect of parathyroidectomy on scleroderma (Bernheim and Garlock, 1939).