An international consensus document on acellular matrix products in the treatment of hard-to-heal wounds such as diabetic foot ulcers, venous leg ulcers and pressure ulcers.
Currently there is no definitive paper or guideline on the use of acellular matrices in acute and chronic wounds. To begin to address this, an expert working group convened in New York, USA in July 2010 to review current knowledge of acellular matrices and their rationale for use.
The recommendations in this document are based on the consensus opinion of the group and the available evidence. They aim to help both generalist and specialist clinicians decide when to use and how to select an appropriate acellular matrix. This document also aids understanding of how these products may be classified within the rapidly growing range of tissue-engineered products that are indicated for wound healing.
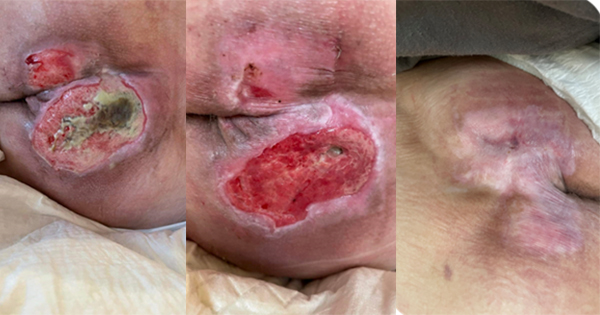
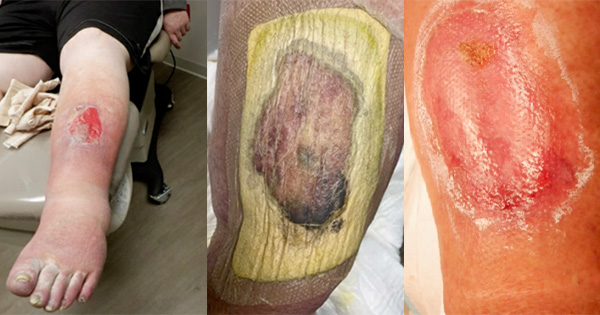
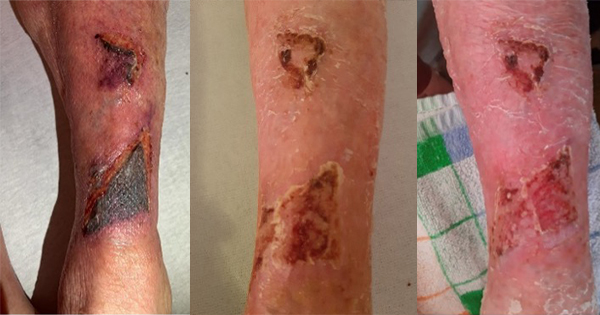
Acellular matrix products can be used in a wide variety of applications, including burns and reconstructive surgery, soft tissue and abdominal wall repair and as internal implants for orthopaedic use in joint resurfacing and tendon repair. This document focuses on the use of acellular matrices (or scaffolds) in hard-to-heal wounds such as diabetic foot ulcers, venous leg ulcers and pressure ulcers.
Supported bt Synovis Life Technologies