Cutaneous wounds are defined as a ‘disruption of normal anatomic structure and function’ of the skin due to an injury/break (Chhabra et al, 2017). MCWs develop when cancer cells infiltrate and proliferate within normal skin and surrounding tissues. These wounds mostly occur either due to metastatic growth of a primary skin cancer or skin invasion by an underlying tumour. The result may be an ulcerating wound or fungating wound (appearance of cauliflower-like protruding tissue) or elements of both (Starace et al, 2022). Sometimes, chronic wounds can also become malignant; Marjolin ulcers are cancers, commonly squamous cell carcinomas, found in previously healed tissues such as old burn scars. The risk of malignancy is variable depending on wound type; these are estimated to occur in 0.5% of vascular ulcers and approximately 1–2% of burn scars (Houlihan et al, 2021).
However, the majority of malignant wounds are a result of primary, secondary or recurrent cancers (Alexander, 2009). An estimated 5–10% of people with cancer are affected by MCWs (based on small-scale surveys), although the full scale of the problem is unknown (Meaume et al, 2013). Tilley et al (2021) found that malignant fungating wounds occur in up to 14% of all advanced cancer patients, with breast cancer (66%) and head and neck cancers (24%) forming the majority (Tilley et al, 2021). Vardhan et al (2019) estimated the prevalence of malignant fungating wounds as 5–14% of all advanced cancer patients in the United States (Vardhan et al, 2019). In one Swiss survey conducted among 269 nurses, the respondents reported a prevalence of fungating malignant wounds in 6.6% of cases; the most frequently affected areas were the breast (49.3%), neck (20.9%), chest (17.6%), extremities (16.6%), genitalia (16.6%) and head (13.5%) (Probst et al, 2009). These numbers may be underestimated since no population-based register exists to follow the incidence of MCWs (Furka et al, 2022).
MCWs can grow rapidly and are associated with malodour, exudate, oedema, necrosis, bleeding, pruritis and infection. In particular, malignant wounds often generate excessive exudate, due to alterations in cellular perfusion and the production of vascular permeability factor (Dvorak, 2015). Where there is necrotic tissue, there may be bacterial growth that activates proteases, causing tissues to liquefy and generate exudate (Starace et al, 2022). Due to exudate discharge, malodour and bleeding, MCWs can be very distressing for patients and those close to them, with patients often facing significant pain and social isolation. There are limited treatment options and prognosis is generally poor (Furka et al, 2022). While data on quality of life (QoL) are limited, one study conducted in Taiwan indicates that patients with MCWs have the lowest possible levels of QoL (Lo et al, 2012).
Due to their complex needs, people with MCWs require a comprehensive, multidisciplinary approach to treatment, encompassing specialist oncologists, surgeons and palliative caregivers, and incorporating pain management and psychosocial support (Furka et al, 2022). A multidisciplinary view of this treatment is depicted in Figure 1. As a part of a multidisciplinary treatment approach, palliative wound care for patients with MCWs should focus on symptom management and protecting comfort and dignity, without necessarily targeting healing (Sezgin et al, 2023). When healing is no longer the goal, palliative treatment revolves around controlling symptoms, both physical and psychosocial, and improving patients’ QoL (Cornish, 2019).
Wound clinicians seek a balance between clinical needs, expected clinical outcomes, and the patient’s own needs and preferences, ultimately striving for ‘wound balance’ (Garten et al, 2023). This focus on the patient’s individual goals — alongside expected clinical outcomes — remains critical when choosing a suitable wound dressing for MCWs.
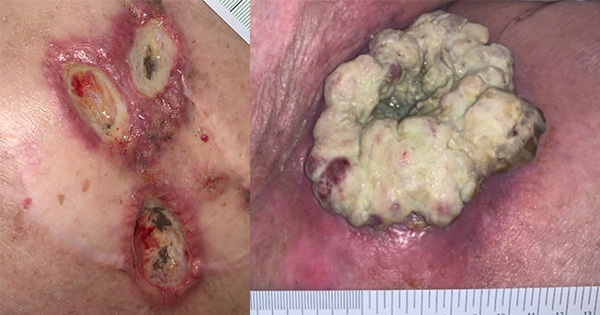
Wound dressings may not always address the unique challenges posed by excessive exudate output, as experienced with MCWs, the presence of which can lead to moisture-associated skin damage, decreased patient comfort, and subsequently, lower QoL (Cullen and Gefen, 2023). Due to this QoL impact, the complicated surface presentation and the fragility of skin around an MCW [Figure 2], there is a significant need in this patient population to provide practical dressings that are atraumatic in application and removal, reduce wound-related pain and reduce potential for bleeding in friable tumour tissue; together, these qualities can improve QoL outcomes in this population by managing exudate, pain
and malodour, and reducing the risk of skin damage.
Silicone dressings have been shown to reduce dressing adherence to wounds and improve outcomes in a variety of wound types (Meuleneire and Rücknagel, 2013; LeBlanc and Woo, 2022; Gefen et al, 2024). Furthermore, recent advances in polymer science have led to the development of superabsorbent dressings that can absorb large amounts of fluids and can help manage the high exudate levels and malodour issues associated with MCWs, the two major issues that are likely to impact life activities for a person living with a cancerous wound (Ousey et al, 2013; Schultz et al, 2019; Singh et al, 2022; Gefen et al, 2024). Superabsorbent dressings are designed to absorb medium to high levels of exudate, maintaining an ideal moist wound healing environment (Browning et al, 2016). These dressings are able to absorb up to 100 times their own weight, and bind and sequester potential wound inhibitors inside the core of the dressing, to avoid further damage to the tissue (Eming et al, 2008).
Silicone SAP dressings combine these two beneficial dressing characteristics and can help achieve the palliative target of protecting patient dignity and comfort (Sezgin et al, 2023).
Keeping this ultimate patient goal in mind, we conducted this case series study by implementing a Quality Improvement Project (QIP) in a tertiary care setting to assess the effectiveness of silicone SAP dressings in managing MCWs. Our aim was to assess silicone SAP dressings for topographically challenging wounds by recording the impact on patients’ QoL and on factors associated with ease of use in routine tertiary care; we also compared the performance of silicone SAP dressings versus the dressings previously used in our setting.
Aim
To evaluate the performance of silicone SAP dressings in patients with MCWs.
Methods
This case series was undertaken as part of a QIP. The author observed and recognised the unmet need that the composite dressings used in this study could fulfil to improve patient outcomes in MCW management; for new dressings to be made available on the hospital dressing formulary, a formal evaluation was required via a QIP. This QIP was based on the quality in healthcare ‘Plan, Do, Study, Act’ methodology (Taylor et al, 2014). A formal review of the silicone SAP dressing* evaluation QIP was submitted to and approved by the Clinical Practice Director and formally lodged in the hospital’s QIP register. Data collection tools were developed from a QIP focus group meeting with senior cancer ward nurses and Nurse Consultants to ensure the tools’ validity and refinement. These tools included a master list tracking trial dressings to patients, and a product evaluation tool. The product evaluation tool was de-identified for patient privacy and included wound bed type (fungating/protruding outwards, or ulcerative/concave inwards, or both). Likert scale-scored questions regarding the product evaluation included: patient wound comfort, ability to stay in place, peri-wound skin condition, atraumatic dressing change, overall clinical impression of dressing performance, ability to contain odour, exudate management, achieving local clinical goals and dressing performance compared to products previously used in our clinical setting; this evaluation also required nurses to provide comments on any advantages observed over previously used dressings, whether the nursing staff would use the dressing on other patients and if they would recommend the dressing to other clinicians. Both tools were initially trialled via 2 patients with MCWs (not included in this study’s data) with nursing staff’s feedback and suggestions incorporated into improving the tools.
The QIP was implemented in four phases. Phase 1 included nursing staff’s feedback on current practices and tool development, focusing on challenges in using current dressings for MCWs. Phase 2 consisted of educating the nurses involved in this study on how to use the study tools and the study products. In the third phase, patients/next of kin (NOK) were offered the use of the silicone SAP dressings, and study tools were used by nursing staff at the point of care to capture outcomes. In the fourth and final phase, data analysis was performed to assess outcomes on the study parameters. One patient’s case was recorded to be presented as a vignette. Throughout the study, staff education and posters were consistently used to remind staff of the
ongoing QIP.
Inclusion criteria: Patients with MCWs with moderate-to-high exudate output, and a wound size that would be covered by trial products’ dressing size, were included in
the study.
Exclusion criteria: Patients/NOK declining the use of trial products were excluded from the study.
Patients who met the inclusion criteria were offered the trial dressing as an option for their routine wound care. Patients/NOK were able to decline participation as per the QIP. All data were de-identified at the point of collection. We used a hospital network talent release form signed by patients/NOK for deidentified wound images, along with network protocols. Application for Ethics Approval for publication was submitted to the institution’s Human Research and Ethics Committee (the Central Adelaide Local Health Network, Ethics Committee; approval number 18804).
Participants
Thirteen patients with MCWs met the study criteria (7 females and 6 males; age range: 41–84 years). The majority of patients were inpatients (12 of 13) with 6 different cancer types: squamous cell carcinoma (53.8%), breast cancer (16.4%), melanoma (7.7%), Ewings sarcoma (7.7%), lymphoma (7.7%), and basal cell carcinoma (7.7%). Of the 13 patients, 4 patients had an ulcerative wound bed type, 6 patients had a combined ulcerative and fungating wound bed type and 3 patients had a fungating wound bed type.
Case vignette: history and assessment
A vignette of an exceptionally-distressing-to-the-patient and hard-to-dress wound was also included in the results as an example of the impact of the silicone SAP dressing.
Patient background
This patient was a 78-year-old female, living alone, who maintained independence in activities of daily living and mobility, with the support of a daughter living interstate. She had a history of hypertension, as well as a previous non-melanoma skin cancer. She had allergies to penicillin, cephalexin, sulphonamides, and clarithromycin, and took no regular medications. She was a non-smoker (current), albeit with a previous 35-year smoking history.
Wound history
In June 2022, the patient attended her local doctor for a growth on her right cheek, which was ‘burnt-off’ on two separate occasions, and grew back each time.
The lesion had doubled in size by June 2023, when she presented to her local doctor again. The local doctor referred her to the Plastics & Reconstructive Surgery Unit of the tertiary hospital, where she was seen as an outpatient in early July 2023. At this appointment, she had a wound biopsy, which led to diagnosis of moderately differentiated squamous cell carcinoma. Magnetic Resonance Imaging was performed and a Head and Neck Cancer Multidisciplinary team review undertaken. Due to extensive spread of the wound, the patient was not recommended for surgical intervention.
In August 2023, the patient was admitted to the tertiary hospital with falls, cognitive decline, acopia and lower-limb cellulitis. The patient was provided with a course of radiotherapy, which shrank the wound significantly.
She later moved into a residential care facility, with palliative care implemented, and passed away on 7th February 2024.
Wound assessment
The wound was a large ulcerative right-cheek lesion (moderately differentiated squamous cell carcinoma) with extensive skeletal involvement and extension to the right nasal ala, right upper lip to the oral commissure, and down to the maxilla.
Wound parameters recorded on 15 August 2023 were as follows: tissue, necrotic malignant wound tissue; wound topography, both fungating and ulcerative; infection/inflammation, some odour present, which reduced with use of a super oxidised hypochlorite solution spray as a soak; moisture balance, moderate seropurulent exudate with some surrounding skin irritation; edges/surrounding skin, scabs and dried blood present; patient was unable to tolerate wound cleaning; wound-related pain, MCW-related allodynia and hyperaesthesia present (Pain Assessment in Advanced Dementia [PAINAD] scale score, 6/10).
Wound dimensions were measured as: length, 8cm; width, 6.5cm; height, 1.5cm. Depth of the wound was recorded as: an oro-cutaneous fistula with teeth on view in the base of the wound.
Wound management goals
Although the patient wanted the wound to be covered, she initially refused all dressings due to fear of pain upon removal (nursing staff at another facility had applied a dry gauze pad and tape to the wound, which led to issues with severe pain on removal, as reported by her daughter).
The patient reported being concerned people were staring at the wound, and was also observed mopping up exudate and saliva with tissues.
The patient-centred wound management goals were as follows: atraumatic dressing on application and removal; reduced local pain with dressing in situ; the wound to be covered to stop people staring; contain exudate.
Results
Overall dressing performance
In total, 26 nurses participated in this case series, with a total of 41 dressing evaluations completed in 13 patients. We report below the outcomes of these dressing evaluations. n denotes the total number of evaluations reported for each measure.
In the majority of evaluations, nursing participants rated the silicone SAP dressings as either ‘excellent’ or ‘good’ in terms of the following parameters related to dressing performance. Patient wound comfort during dressing wear time (n=39/41) was excellent in 31% and good in 49% of reported evaluations; ability to stay in place (n=39/41) was excellent in 23% and good in 35% of reported evaluations. Peri-wound skin condition (n=38/41) was excellent in 18% and good in 55% of reported evaluations. Ease of removal/atraumatic dressing changes (n=39/41) was excellent in 38% and good in 48% of reported evaluations. Dressing integrity (i.e. ability to maintain form and function during wear time; n=38/41) was excellent in 42% and good in 28% of evaluations.
For 29% of all evaluations, the overall clinical impression of dressing performance was rated as ‘excellent’ and, in 50% of all evaluations, respondents gave an overall impression rating of ‘good’ (n=38/41). A summary of all responses related to dressing performance is given in Figure 3.
Management of exudate and odour
For exudate management, respondents were asked whether exudate was contained within the dressing, leaking from the dressing, or damaging the skin. In the vast majority of reported evaluations (92%), exudate was contained within the dressing. For the remaining 8%, exudate was leaking, with none recorded that the exudate was damaging the skin (n=37, missing data=4).
Respondents were also asked to review the ability of the dressing to contain odour for each evaluation. For 68% of reported evaluations, there was no odour, while for 32%, there was some odour. Importantly, presence of malodour was not noted in any evaluations (n=37, missing data=4).
Local clinical goals, related to exudate management and protection of delicate peri-wound skin and vulnerable areas, were either fully (72%) or partially (28%) met in all reported cases, as shown in Figure 4 (n=36, missing data=5).
Comparison of silicone SAP dressings with previously used products
When asked to compare silicone SAP dressings with the previously used products, for 58% of evaluations, the silicone SAP dressings were rated as ‘better’ than the previously used product; for 16%, they were ‘similar’; for 3%, they were ‘inferior’; in another 24% of evaluations, respondents were unable to answer, as they had not seen the effect of the previous product, or there was no product previously in place (n=38, missing data=3).
Advantages of silicone SAP dressings over previously used dressings were provided in 27 free hand-written comments and impressions from participating nurses, as listed in Table 1.
Intentions for further use of silicone
SAP dressings
In the majority of evaluations (92%), respondents stated that they would consider using silicone SAP dressings for other patients (n=38, missing data=3), and that they would recommend them to other clinicians (92%) (n=39, missing data n=2).
Case vignette outcomes
As evident from the wound assessments, this patient had a particularly challenging MCW that, along with a complex topography, was located on the mouth of the patient, causing huge distress and QoL complications. Figure 5 shows the MCW progression for this patient and the level of coverage provided by the silicone SAP dressing.
Outcomes achieved with silicone SAP dressing
The patient’s wound management goals were achieved using a silicone SAP dressing, which included atraumatic application and removal, coverage of the wound, and exudate being contained within the dressing. PAINAD score was 1/10.
The patient reported: “People no longer stare at the wound as I have a dressing in place”, and stated that “the dressing was soft and comfortable”. Her daughter added: “We want to continue to use the dressing as it makes her feel better, with no pain” and “such a small thing as a dressing can make a big difference – that dressing really helped”.
Discussion
This case series assessed the outcomes of silicone SAP dressing on MCWs with challenging topographies, recorded via evaluation of nurses’ responses on wound dressing outcomes.
Despite the complexity of wounds and the variety of cancer types, there were several positive patient outcomes recorded in this study, reflecting the achievement of balanced management of MCWs and protection of patient comfort and dignity (Sezgin et al, 2023). Firstly, the use of a silicone SAP dressing achieved ‘excellent’ or ‘good’ patient wound comfort in 80% of evaluations. In MCW management — a predominantly palliative intervention — the wound pain may be due to a number of issues, such as the tumour pressing on other body structures, damage to the nerves by the growing tumour, swelling resulting from impaired capillary and lymphatic drainage, infection, exposed nerve endings and even poor dressing change technique (Woo and Sibbald, 2010). It may also be related to moisture-associated skin damage from leaking or pooling exudate in the surrounding skin and skin folds. In the vast majority of our patients, pain management to a good level indicated the potential of silicone SAP dressings in providing comfort to patients with MCWs.
Secondly, for the majority of evaluations, respondents noted that exudate was contained within the dressing. Appropriate exudate management in MCWs is essential for patient comfort and dignity, and can also lead to decreased odour (Graves and Sun, 2013). MCWs can produce up to one litre of exudate per day (Starace et al, 2022) which needs to be contained via dressings. This containment also reduces peri-wound skin damage (Weir, 2012) and fear of embarrassing odour and exudate strikethrough (Probst et al, 2013).
Thirdly, respondents in the majority of evaluations rated the dressings ‘excellent’ or ‘good’ for atraumatic dressing changes – i.e. patient comfort during dressing change. Given that MCWs are often extremely painful and bleed easily (Naylor, 2001), a dressing that is atraumatic and comfortable is advantageous. The majority of nurse responders also noted they would recommend silicone SAP dressings to other clinicians for MCW management through their experience with this project; this highlights the positive impact of silicone
SAP dressings on nurses’ motivation when performing palliative dressing routines.
Finally, the majority of dressings were described as ‘excellent’ or ‘good’ in terms of ability to stay in place, even in very difficult-to-dress areas, which is often the case with MCWs (Starace et al, 2022). Most of the dressings used in this study were bordered although, during staff product education sessions, this was strongly advised against in use on fungating wound presentations. In fact, the majority of wounds in this study had either a combined ulcerative and fungating wound bed or a fungating wound bed, which may have impacted the ability of the dressing to stay in place in some cases. Indeed, where leakages were noted, it could potentially be explained by the challenging topography of those specific wounds. In addition, presence of odour may be related to the ability of the dressing to contain exudate given specific wound topography, since leakage often equals odour. The presence of a soft absorbent pad with an atraumatic interface may have helped with contouring to the wound and providing comfort. Use of an algorithm chart for dressing choice based on MCW topography may be useful to support implementation of similar dressing techniques in hospital or tertiary care settings.
Wound management of patients with MCWs poses substantial challenges and is often more complex than the care of other chronic wounds (Probst et al, 2009). This complexity arises from tissue presentation, wound topography, wound pain, high exudate levels, malodour, excessive bleeding and the development of peri-wound skin moisture-associated skin damage. Therefore, the overall aim for wound management was not to heal but to protect the patient’s comfort and dignity (Probst et al, 2009; Sezgin et al, 2023). The focus was to achieve wound balance and protect the comfort and dignity of our patients by ensuring that the undulating wound tissue topography was an essential consideration for each dressing selection. Dressings that conform, are soft and pliable enough to wrap around fungating tissue, and maintain intimate contact with the wound are essential to prevent exudate pooling and leakage (Weir, 2012). Furthermore, fungating, ‘cauliflower-like’ lesions with protruding tissue and wound height impact the decision to apply an adhesive-edged versus a non-adhesive-edged silicone SAP dressing. In this backdrop, we also recorded an important, though observational, finding: a fungating wound with height requires stronger fixation with polyacrylate tape rather than silicone or film edging, due to both wound topography and body movement (n=2; as reflected in nursing staff comments).
Overall, the results demonstrate that silicone SAP dressings make a positive difference to patients’ QoL and wound outcomes in malignant wound care, even when wound healing is no longer the primary goal; in addition, a majority of tertiary care nurses preferred the silicone SAP dressings over previously used dressings for managing MCWs. As a result of performing this study via a QIP, the team was able to recommend that silicone SAP dressings are made available within the hospital’s wound dressing bank for use with patients with MCWs. It also highlighted the need to educate tertiary care clinicians on how to best use silicone SAP dressings to achieve both expected clinical outcomes and meet patient needs and preferences. To further improve the function of silicone SAP dressings, we recommend integration of an internal charcoal absorbing layer within the dressing, or as part of the dressing’s interface.
Limitations
This case series was open-label and was based on nurses’ observations through completion of paper-based user product evaluation forms (our data collection tool). Therefore, observation bias is a limitation of our study. Furthermore, instrument reliability was not formally tested due to the study being an unfunded QIP conducted in addition to usual work requirements for the clinicians taking part in the study. Small patient numbers (n=13) and a small data tool pilot (n=2) were also limitations to this project; however, the tools were reviewed for content validity by 5 senior nurses experienced in MCW management, with pilot feedback incorporated into the tools.
Further studies in a larger number of patients should be conducted to confirm outcomes noted in this case series, with tools more rigorously tested on different types of MCWs. The MCWs observed in this case series study were challenging due to high exudate levels, with fungating tissue and/or ulcerative cavities.
Conclusion
Achieving ‘wound balance’ as the focus of patient-centred MCW management is vital in protecting patient comfort and dignity. Furthermore, wound topography should be considered in selecting wound dressing type (silicone-edged self-adhesive versus non-adhesive) for MCWs. This case series evaluated feedback from tertiary care nurses on the use of silicone SAP dressings for MCWs. The study outcomes support the effectiveness of silicone SAP dressings in management of MCWs of varying types and locations. Our results also suggest that tertiary care clinicians may prefer to use silicone SAP dressings because they manage exudate, are atraumatic in application and removal, and lead to positive feedback from both patients and nurses, indicating improved QoL outcomes.
Acknowledgements
The author would like to thank colleagues who supported this QIP, including: Dr Rebecca Munt, Research Nurse, Nursing Education CALHN/University of Adelaide for the review of the data collection tools and QIP ethics screening; Ward Nurses involved in the project; Head and Neck Cancer Nurse Consultant Caroline Whitford; Breast Care Nurse Consultants Trisha Harris and Catherine Lawson; Ward Nurse Unit Managers Melissa Keighran, Kirsty Howell, and Gabrielle Vigar; HARTMANN for trial products and for providing product education to staff; the patients who agreed to participate in the project; the case vignette patient and her NOK for permission to use her story and images.
*Zetuvit® Plus Silicone Border is the HARTMANN brand for the tested silicone SAP-containing dressings