Wound healing is a multifaceted biological process encompassing haemostasis, inflammation, proliferation, and tissue remodelling, all of which must occur in a coordinated manner to restore skin integrity and function (Singh et al, 2017). In particular, the role of dermal fibroblasts in generating connective tissue and producing the extracellular matrix had been highlighted as essential for skin recovery (Hannen et al, 2023; Novis and Takiya, 2024).
The management of severe wounds remains a significant clinical challenge due to the risk of complications such as infections, necrosis, and delayed healing, which can result in prolonged hospital stays and increased healthcare costs (Järbrink et al, 2016). Of some concern though, is that conventional wound care approaches often fall short in cases involving extensive tissue damage, chronic wounds, or comorbidities, such as diabetes. The eradication of bacteria is also a necessary prerequisite for wound healing (Kaufman et al, 2018). Commonly used iodine-based disinfectants such as povidone iodine have been widely studied (Bigliardi et al, 2017a, 2017b; Barreto et al, 2020), but challenges persist in managing non-healing wounds.
Aiodine™, a novel iodine-based formulation developed by Aiodine Laboratory Pte Ltd, represents an innovative solution with the potential to address these challenges. Prior to this study, in vitro tests conducted under stringent EN standards demonstrated its broad-spectrum antimicrobial efficacy against both Gram-positive and Gram-negative bacteria, with a log 5 and greater reduction at contact times of 30 seconds. This antimicrobial activity is crucial for minimising infection and potentially supporting wound healing. It is hypothesised that Aiodine™ enhances the wound microenvironment, fostering conditions conducive to accelerated tissue repair.
This study sought to assess the efficacy and safety of Aiodine™ in the treatment of severe wounds. By examining its performance in a clinical setting, this research aims to provide preliminary evidence supporting its therapeutic potential and lays the groundwork for future large-scale investigations. Additionally, the study briefly explores how Aiodine™ compares to other commonly used disinfectants and its efficacy against antibiotic resistance infections (Lai et al, 2020).
Study objectives
The primary objective was to evaluate the efficacy of Aiodine™ in accelerating wound healing in patients with severe wounds. The secondary objectives were to assess the safety and tolerability of Aiodine™ when applied to patients; investigate the impact of Aiodine™ on reducing infection rates, inflammation, and pain at wound sites; and assess quality-of-life improvements related to wound healing, utilising validated questionnaires.
Methods
This was a pilot intervention study (clinical trial). The design was an open label, single-arm study evaluating initial efficacy and safety.
The treatment duration was determined based on individual healing progress, followed by a a-week safety monitoring period. Aiodine™ was applied directly without dilution to ensure treatment consistency.
This study was conducted at the Wound Department of the First Affiliated Hospital of Hainan Medical University, Haikou, China. All patients gave verbal consent before treatment with Aiodine™.
Inclusion criteria were patients aged 18 years or older with wounds classified as severe by attending clinicians. Wounds were chronic refractory wounds persisting for at least 2 weeks without significant improvement. Wounds ranged in size from 1–10 cm² in area. Adequate blood flow to the affected limb was confirmed using an ankle-brachial index (ABI) >0.7 or toe-brachial index (TBI) >0.5.
Exclusion criteria:
- Active or suspected infections requiring systemic antibiotics.
- Gangrene or ischaemic wounds.
- Known hypersensitivity to iodine or related compounds.
- Use of other investigational wound-healing agents within the past 3 months.
Study population
Four patients with clinically diagnosed severe wounds, varying in aetiology and size, were recruited. The participants were adults aged 42–66 years with severe wounds characterised by:
- Soft tissue necrosis (post-ant bite).
- Diabetic foot and gangrene (following amputation).
- Stage 4 pressure injuries (post-surgery).
- Purulent paronychia.
Intervention and assessment
Wounds were cleaned and debrided as necessary before each application, following standard care protocols. Aiodine™ was topically applied once daily for up to 2 weeks, either as a spray or wet compress, tailored to each patient’s healing response. Daily assessments included:
- Wound healing progress (e.g. reduction in wound size).
- Signs of infection or inflammation in surrounding tissues.
- Adverse events, including patient-reported discomfort.
Statistical considerations
The small sample size limits generalisability, but we observed general visual healing, with the patients reported as feeling less uncomfortable and feeling significantly less pain. Future trials will aim to recruit larger cohorts for more robust statistical analysis.
Measuring statistical significance with only four patients is not possible, because it is such a small sample size.
Case 1
A 57-year-old man bitten by ants 1 month ago. He presented with skin ulceration and purulent discharge on the right anterior lower leg calf for more than 1 month, worsening with fever for 1 day.
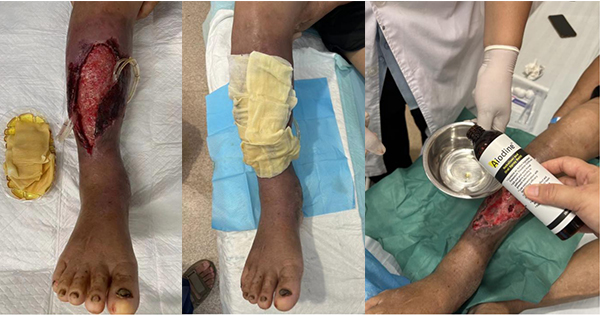
Physical examination showed obvious swelling of the right calf and foot, moderate pitting oedema, about 6 cm × 4 cm, in the anterior right lower leg. The wound had a large amount of purulent secretions, foul odour, tenderness,and obvious redness and swelling around [Figure 1a and 1b].
Suspected soft tissue infection of the right anterior lower leg was confirmed by bacterial culture, which identified a drug-resistant strain of Pseudomonas aeruginosa. The wound’s severity necessitated a skin graft. We used Aiodine™ wet application to completely eliminate the bacteria, then we performed skin grafting surgery to repair the wound.
Treatment
An Aiodine™ wet compress was applied to eradicate the P aeruginosa pre-grafting. The wound discharge cultures were negative after 1 week of applying Aiodine™.
After the skin graft, a clean Aiodine™ wet compress was used [Figure 1c and 1d]. The skin graft was successful, and subsequent bacterial cultures remained negative.
Healing and observations
At 7 days post-surgery: The wound showed significant healing [Figure 1e and 1f], and bacterial cultures continued to test negative. Aiodine™ usage was discontinued. The dramatic improvement from the pre-graft state to post-graft healing is noteworthy. Tubing drainage prevented purulent secretions from accumulating in the cavity space and further aggravating the infection.
The findings suggest that Aiodine™ did not impede healing, indicating it is not cytotoxic to healing tissue or grafted skin. Instead, it is likely that it facilitated the healing process by controlling the infection, allowing the skin graft to take. A skin graft heals through a three stage process: imbibition, inosculation and revascularisation.
Additional observations
Similar positive outcomes were noted in another incidental case involving an Indonesian male who self-administered Aiodine™ for a deep purulent wound [Figure 2a and 2b], with no other medical interventions provided. For most of the time, no bandage was applied to the wound. The Aiodine™ formulation was sprayed directly into the wound area and repeated several times a day. When a bandage had to be used after several days because the patient had to travel to a different location, the bandage was soaked in Aiodine™ and directly sprayed into the wound area. In this instance, the bandage stayed on for close to a day. When the bandage was subsequently removed, no skin or flesh was reported to be removed. It was noted that at no point in time was there a foul odour from the wound. This reinforces the potential effectiveness of Aiodine™ in enhancing wound healing and managing infections.
Case 2
A 42-year-old man who had undergone leg amputation for diabetic foot ulcer and infection of the left foot and left leg. The patient had type 2 diabetes with peripheral vascular disease and peripheral neuropathy.
The stump of the left leg was ulcerated for more than 1 month after amputation, and it worsened, accompanied by fever for 3 days.
The stumps of the left tibia and fibula were exposed, the outer part of the upper tibia was exposed. The wound had a small amount of necrotic fascia and adipose tissue, and a large amount of granulation tissue growth [Figure 3a and 3b]. Surgical debridement was performed to clear the necrotic tissue. Following debridement, the stump was closed primarily. Treatment was with an Aiodine™ wet compress applied to the affected area.
Healing
After 5 days of treatment, there was a noticeable reduction in both redness and swelling [Figure 3c]. At 14 days post-operation, the sutures were removed, and the incision had healed well, indicating effective management of the wound [Figure 3d]. Surgical debridement was performed to clear the necrotic tissue. The exposed end was detached.
Observations and conclusions
This case demonstrates that Aiodine™ was effective in controlling infection and reducing inflammation where the patient had long-term diabetes, lower limb vascular occlusion and neuropathy, and severe diabetic foot infection. The first leg amputation incision was infected, and the second leg amputation was performed. Meanwhile, we used external Aiodine™ to prevent incision infection and the incision healed well. (Part of the incision edge appeared swollen.) The quick healing observed suggests that Aiodine™ not only stemmed the infection but also exhibited a lack of cytotoxicity, facilitating and potentially accelerating the healing process. The positive outcomes reinforce the formulation’s utility in post-operative wound care.
Case 3
The patient was a 66-year-old man with haemorrhagic stroke with spastic hemiplegia, cognitive impairment and dysphagia. He also had hypertension (stage III, very high risk), hypoproteinaemia, fatty liver, diffuse emphysema of both lungs, and impaired glucose tolerance and ad undergone prior above-the-knee amputation.
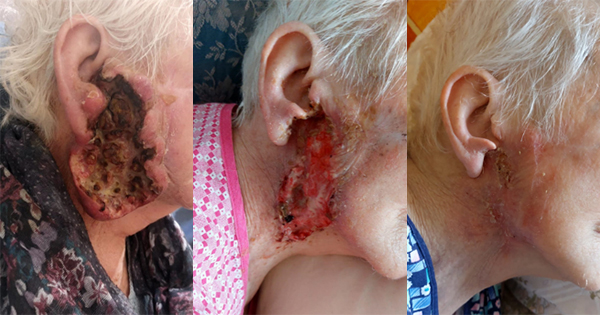
He had necrosis of the left femoral head with ulceration and purulent discharge of more than 1 year’s duration, aggravated with fever for more than 1 day.
Physical examination: A wound of about 2cm × 2cm on left trochanter, with two drainage strips left, redness and swelling around the wound edge, and a little exudate [Figure 4a and 4b]. The drainage pipe passes through the subcutaneous space, and we pumped the pipe back and forth every time when the dressing is changed, and the fluid flows out from around the pipe mouth.
The diagnosis was stage 4 trochanteric pressure ulcer with infection.
The rapid healing in this case with no signs of infection or complications, can likely be attributed to the effective disinfection following Aiodine™ application, and care provided [Figure 4c]. This suggests that proper wound management and infection control play crucial roles in promoting healing in surgical patients.
Case 4
A 55-year-old woman presented with ulceration and pus discharge on the right thumb of more than 1 week’s duration.
Physical examination revealed swelling of the right thumb, about 3 cm × 2 cm in size, with a large amount of purulent secretions, foul odour, tenderness, obvious redness and swelling around, and partial ulceration of the nail bed [Figure 5a].
The diagnosis was suppurative paronychia, which is typically caused by antibiotic-resistant Staphylococcus aureus. However, the exact aetiology was not investigated in this instance.
In this case, the patient underwent nail extraction and drainage. The affected area was cleaned and disinfected with Aiodine™, followed by treatment with a wet compress. For this case, contact with the patient was lost and there was no follow-up to gauge the further progression of wound healing.
Results
The study demonstrated significant improvements in wound healing across all four cases, with no significant discomfort reported by patients.
Discussion
Aiodine™ demonstrated promising efficacy in wound management, particularly against antibiotic-resistant bacteria, without evidence of cytotoxicity, distinguishing it from other commonly used disinfectants, such as chlorhexidine acetate, and polyhexamethylene biguanide. Although examined under different study conditions, both have been associated with cytotoxic effects that may hinder wound healing (Wang et al, 2022 Zhang et al, 2023). However, for another common disinfectant, povidone–iodine, Zhang et al (2023) and Wang et al (2022) had observed cytotoxic effects, but Bigliardi et al (2017a, 2017b) did not concur with those observations.
Mechanistically we can expect that Aiodine™ will work in a similar fashion to povidone–iodine, whereby the presence of free iodine (Bigliardi et al, 2017a, 2017b) oxidises the pathogen nucleotides and fatty /amino acids and thus deactivates protein as well as DNA/RNA. However, unlike povidone–iodine (a synthetic water-soluble polymer), Aiodine™ is a natural extract, with additionally other complex hydroxy iodine and polyiodic species.
While these results with Aiodine™ are encouraging, the small sample size necessitates further research to statistically validate the findings. Future studies should include randomised, double-blind, placebo-controlled trials with larger cohorts to confirm the efficacy and safety of Aiodine™. Additionally, longer follow-up periods will help to better evaluate its impact on sustained wound healing outcomes.
Conclusion
This case series highlights the potential of Aiodine™ to address critical challenges in wound care, particularly in cases involving severe wounds and antibiotic-resistant infections. The findings from this case series suggest that Aiodine™ could serve as a valuable addition to wound care protocols, offering a potentially safer and more effective alternative to existing disinfectants.