Clinical treatment of chronic wounds is an ever-evolving practice, largely consisting of multiple modalities including mechanical and enzymatic debridement, washing of wounds, and disruption of microbial or fungal colonisation to allow for re-epithelialisation (Velnar et al, 2009). In the last decade, researchers have implicated microbial biofilms as a key impedance in the successful healing of chronic wounds and has prompted the development of several anti-biofilm agents as new therapies in healing chronic wounds (Sen et al, 2021).
One recently developed anti-biofilm agent is BlastX gel (Next Science), approved by the US Food and Drug Administration (FDA) in 2017. This product is an odourless gel designed to maintain a moist wound environment and consists of polyethylene glycol-based hydrogel with a pH buffer system and benzalkonium chloride surfactant (Serena and Jalodi, 2021). This chemical makeup is proposed to destabilise biofilms by chelating calcium and removing proteins from the bacterial cell membranes, ultimately weakening extracellular polymeric substances (EPS) that maintain the biofilm. With a weakened EPS, the bacteria are then exposed to benzalkonium chloride in the BlastX, which lyses the cell wall, resulting in the dissolution of the biofilm and destruction of the bacteria that colonise the area (Serena and Jalodi, 2021).
This case report describes a patient who presented to our wound care centre with chemotherapy-induced eruptive pustulosis (EP) of the scalp. Previous dermatological treatment included regular in-office debridement and topical steroid creams as well as alteration of his chemotherapy regimen, with no clinical improvement over the course of 8 months. Upon presentation to our clinic, we initiated a regimen consisting of daily washing with over-the-counter baby-shampoo, BlastX gel, Promogran Ag (3M) and Xeroform (Covidien). Over the next 3 months, he demonstrated total clinical resolution of his EP, demonstrating the efficacy of a multimodal regimen which includes a biofilm-disrupting agent.
Case summary
FM was a 70-year old man maintained on a chemotherapy regimen of carboplatin, pemetrexed, pembrolizumab and amivantamab for metastatic adenocarcinoma of the lung. He developed EP of the scalp and was treated by the dermatology team, who performed regular mechanical debridement, topical washing agents and topical steroids. His oncologist also decreased the frequency of his amivantamab treatments to try to help improve the EP. However the EP was refractory to 8 months of the dermatological treatment regimen described above. He then presented to our clinic.
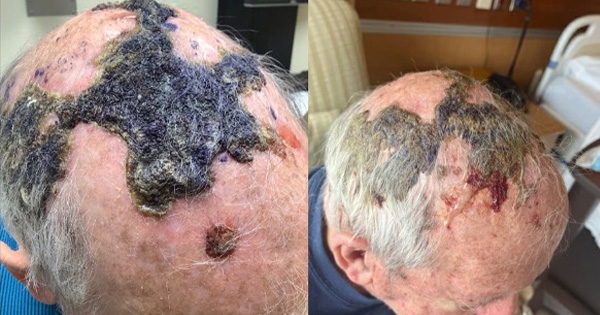
Initial assessment of his scalp showed extensive crusting with embedded hair, keratin slough and several areas of draining purulence [Figure 1]. Approximately half of the crusting was manually debrided, showing superficial epidermal loss under newly debrided areas and the clinical diagnosis of EP was formally assigned.
Promogran AG (3M) and daily washing with lukewarm water was prescribed for the next week while the patient waited for the BlastX gel to arrive. The primary rationale behind the initiation of Promogran AG was the clinical finding of his initial eschars and underlying wound bed being considerably desiccated, necessitating the addition of a moisturising agent to help promote a healthy wound bed for granulation.
On week two of treatment, his scalp had already began to show signs of improvement with significant reduction in crusting, a small amount of advancing epithelium and no purulent lesions [Figure 2]. Mechanical debridement of crust and necrotic tissue was performed. BlastX was provided and the patient was instructed to wash lesions with lukewarm water for 10-15 minutes, and apply BlastX, Promogran AG and Xeroform daily. Promogran AG was continued because the patient noted his wound bed would easily desiccate without the use of this product’s moisturising properties.
On week four of treatment, residual shallow ulcerations of the scalp lesions were noted, with a mild degree of biofilm present. However there was overall less inflammation and no active areas of bleeding or purulence [Figure 3].
Significantly, the patient noted he no longer experienced pain associated with his lesions at rest and his quality of life had improved as a result. He was instructed to continue his regimen but to start washing the lesions with a zinc-based shampoo. However, this was discontinued the next day due to discomfort and he changed to an over-the-counter baby shampoo.
By the sixth week of treatment, the majority of his scalp was completely healed with only few areas of residual open wounds and the patient reported he only continued to have minimal discomfort when performing wound care and no pain at rest (Figure 4). His remaining wounds were completely healed by his third month of treatment with this regimen. The patient transitioned to daily application of Aquaphor (Beiersdorf) to moisturise his scalp and he was discharged from our wound care clinic.
Notably, this wound-care regimen was quite effective for this patient because of its simplicity, empowering a great degree of patient autonomy and confidence in being able to perform his own wound care.
Discussion
This case demonstrates the utility of multimodal therapies including the use of an anti-biofilm agent for EP refractory to traditional dermatological treatments. In comparing the regimens prescribed by dermatology and our wound care centre, the main difference appears to be the discontinuation of topical steroids in favour of BlastX.
Traditional treatment of chronic wounds has classically involved debridement, washing of wounds and topical antibacterial agents (Velnar et al, 2009). Microbe colonisation and biofilm formation has been implicated as a main contributor to chronic wounds and calls for research investigation of biofilm-disrupting agents (Sen et al, 2021).
Overall, there is limited literature which explores the efficacy of BlastX in reducing microbe colonisation. One previous study compared BlastX to multiple antimicrobial agents and demonstrated that across various models which simulate different types/stages of chronic non-healing wounds, BlastX was consistently the most effective anti-biofilm agent in reducing microbial colonisation and promoting healing of wounds (Stoffel et al, 2020). Similarly, a prospective, randomised, open-label trial comparing BlastX to a standard triple-antibiotic ointment demonstrated improved wound healing and lower bioburden in the BlastX group (Myntti et al, 2022).
Additionally, Cox et al (2023) examined the efficacy of BlastX against Candida species by using a zone of inhibition, biofilm and time-kill assays to successfully demonstrate efficacy of BlastX in reducing Candida colonisation. BlastX mechanism of action therefore seems to have applications in reducing bacterial and fungal biofilms.
In comparing the effectiveness of anti-biofilm agents to topical antibiotics, Kim et al (2018) compared the combination of mechanical debridement plus BlastX, to the application of Neosporin+ Pain Relief Cream (J&JCI) over a 3-month period. They concluded there was a 71% reduction in the size of the wound bed in patients receiving debridement plus BlastX, whereas patients receiving Neosporin only demonstrated a 24% reduction in wound area, implicating that biofilm disruption with mechanical debridement and an anti-biofilm agent can allow for speedier wound healing than application of topical antibiotics alone (Kim et al, 2018).
One popular criticism of the use of biofilm-disrupting agents in comparison to topical antibiotics is their cost. Carter and Myntti (2019) employed a Markov microsimulation to evaluate the cost-utility of using a biofilm-disrupting agent compared to a topical maximum-strength triple antibiotic ointment over the course of 4 weeks. These simulations were used to determine cost differences by examining quality-adjusted life years (QALYs) and incremental cost-effectiveness ratios (ICERs). Compared to patients who underwent debridement and Neosporin alone, patients who underwent debridement and BlastX had an ICER of $8,794 per QALY whereas those who failed to have any wound healing with Neosporin after 4 weeks and began receiving BlastX treatment had an ICER of $21,566 per QALY. This study has the implication that initiating treatment with a biofilm-disrupting agent instead of a cheaper, topical antibiotic may ultimately be more cost effective than failing treatment with topical antibiotics and converting to biofilm-disrupting agents later. Additionally, the use of topical anti-biofilm agents instead of topical antibiotics has the advantage of reducing the risk of developing bacterial resistance to antibiotics which has widespread benefits for clinicians and the general population as a whole.
BlastX has also shown promise when used in conjunction with negative pressure wound therapy (NPWT), although this was not used for our patient. In an effort to determine the efficacy of BlastX in conjunction with NPWT for pressure ulcers, Serena and Jalodi (2021) published a case series of six patients. It was found that over the course of 4 weeks using BlastX and NPWT, 66% of patients experienced >20% of closing of their pressure ulcers with an average wound surface area reduction of 49% across all patients. Additionally, by using a MolecuLight Procedure, they discovered a significant reduction in bacterial burden in all wounds with complete elimination of bacterial colonisation in 30% of patients, demonstrating that biofilm-disrupting agents like BlastX can also be a useful adjunct to NPWT (Serena and Jalodi, 2021). Notably, this study highlights the potential efficacy of a biofilm-disrupting agent in conjuction with advanced wound closure devices such as NPWT.
Conclusion
The case detailed in this report supports the previous literature that details more rapid healing of chronic wounds with biofilm-disrupting agents (BlastX in particular) compared to alternative and traditional treatments, both for typical and atypical wounds. Our case study is limited by the fact that multiple wound care agents were utilised simultaneously throughout the course of the patient’s treatment and therefore no direct cause-and-effect can be concluded regarding the effectiveness of any agent alone.
Additional research is needed to continue demonstrating the effectiveness of biofilm-disrupting agents such as BlastX in comparison to other treatment modalities and how concomitant use of multiple wound care agents may affect the individual effectiveness of each individual agent, as well as in combination with advanced wound closure devices such as NPWT. The information presented in this report implicates that the addition of a topical anti-biofilm agent has the potential to accelerate the healing of chronic, non-healing wounds and improve quality of life.