Lymphoedema is a localised accumulation of interstitial oedema due to impaired lymphatic drainage (Warren et al, 2007; Grada and Phillips, 2017; Lee et al, 2022). Generally, lymphoedema is categorised into two subtypes: primary, caused by developmental anomalies of lymphatic channels (Warren et al, 2007; Grada and Phillips, 2017; Brouillard et al, 2021), and secondary, caused by an insult to previously normal lymphatic channels (Warren et al, 2007; Grada and Phillips, 2017; Azhar et al, 2020; Brouillard et al, 2021).
Historical literature dating back to the 1960s involving nuclear medicine studies (Golueke et al, 1989; Goss et al, 2019), and peripheral intranodal lymphangiography (IL) studies (Buonocore and Young, 1965; Edwards and Kinmonth, 1969) via peripheral injection of the dorsal web spaces of the foot, characterise certain patterns that were thought to be more frequently seen with primary lymphoedema. These patterns include pre-existing lymphovenous anatomic anastomoses present in 80% of patients with primary lymphoedema (Goss et al, 2019) versus 50% of patients with secondary lymphoedema (Bains et al, 2015) versus 2–3% of the general healthy population (Edwards and Kinmonth, 1969). Additional findings more prevalent in primary lymphoedema are structural lymphatic abnormalities resulting in filling of collateral lymphatics, delayed clearance of contrast from the lymphatic vessels (Buonocore and Young, 1965), contralateral lymphatic abnormalities (Buonocore and Young, 1965; Golueke et al, 1989), lymphatic aplasia (Buonocore and Young, 1965), and delayed visualisation of the central lymphatics (Buonocore and Young, 1965; Golueke et al, 1989; Goss et al, 2019). Our group recently confirmed these findings using IL, but did not perform a comparison to a contemporary cohort of patients undergoing central intranodal lymphangiography without lymphoedema (Kim et al, 2024).
In this retrospective study at a single-institution tertiary referral centre, we reviewed central IL from the groin or neck lymph nodes to examine the rates of these lymphatic patterns: irregular lymph node morphology/paucity of lymph nodes (Buonocore and Young, 1965), lymphovenous shunting from pre-existing anastomoses between the lymphatic and venous systems (Buonocore and Young, 1965), passage of contrast past midline to the contralateral lymphatics, and delayed/non-opacification of central lymphatics in primary lymphoedema versus control patients.
Methods
This retrospective observational study performed at a single-institution tertiary referral centre included consecutive patients with lymphatic leak who underwent central IL between January 2017 and April 2022. The institutional review board approved this Health Information Portability and Accountability Act-compliant retrospective study (IRB 2022P000586) with a waiver of informed consent.
Patient cohort
A manual search was conducted of the institutional radiology report database for procedures categorised as ‘lymphangiograms’ under the angiography modality performed between January 2017 and April 2022. Patient history was reviewed to confirm a clinical diagnosis of primary lymphoedema or lymphatic leak. All patients with primary lymphoedema had an abnormal nuclear lymphoscintigraphy prior to IL. In addition, all primary lymphoedema patients were evaluated at the institution’s lymphatic centre and given the clinical diagnosis prior to referral for lymphangiography.
Lymphatic pattern selection
Various patterns of lymphatic flow that have been described in primary lymphoedema were identified and collated through a review of the literature from studies dating back to 1965. These patterns were: irregular lymph node morphology/paucity of lymph nodes (Buonocore and Young, 1965), lymphovenous shunting from pre-existing anastomoses between the lymphatic and venous systems (Buonocore and Young, 1965), passage of contrast past midline to the contralateral lymphatics, and delayed/non-opacification of central lymphatics (Buonocore and Young, 1965; Golueke et al, 1989; Goss et al, 2019).
The procedure reports from the lymphangiograms from the primary lymphoedema study cohort were reviewed for the presence of the above radiologic findings. Since lymphangiograms for lymphatic leak may have not included a detailed description of the lymphatic drainage patterns in the procedure report, leading to underreporting of the drainage patterns, a blinded interventional radiologist with 4 years’ experience independently reviewed the intranodal lymphangiograms from the lymphatic leak patients and recorded the presence or absence of the aforementioned lymphatic drainage patterns.
Intranodal lymphangiography
The procedure was performed as previously described by Nadolski et al (2012) under moderate sedation. A 22 G 10 cm spinal needle (Becton Dickinson, Franklin Lakes, New Jersey, US) was introduced into the cortex of inguinal lymph nodes. Aliquots of Optiray 320 contrast (Guerbet, Princeton, NJ, US) were injected into the inguinal lymph nodes to confirm the opacification of lymphatic channels. Lipiodol (Guerbet, Princeton, New Jersey, US) was injected to observe the transit through the lymphatic system under fluoroscopy. Rotational cone-beam computed tomography (CBCT) was performed at the operator’s discretion to help delineate the lymphatic anatomy. Embolisation of the lymphatic channels in the lymphatic leak patients was performed at the discretion of the operator.
Statistical analyses
A Fisher’s exact test was used to compare the frequency of the presence of each given lymphatic pattern of drainage between the primary lymphoedema and lymphatic leak group.
Results
Seventeen primary lymphoedema patients (ages 29–75, 14 women) and 17 patients with lymphatic leak (ages 23–80, four women) were identified for inclusion. Patient demographics are noted in Tables 1 and 2. All primary lymphoedema patients had lower extremity lymphoedema (Table 1), while lymphatic leak patients had lymphatic symptoms including chylothorax, chylous ascites, and plastic bronchitis (Table 2). Five of the 17 lymphatic leak patients were confirmed to have active lymphatic extravasation on IL (Table 2).
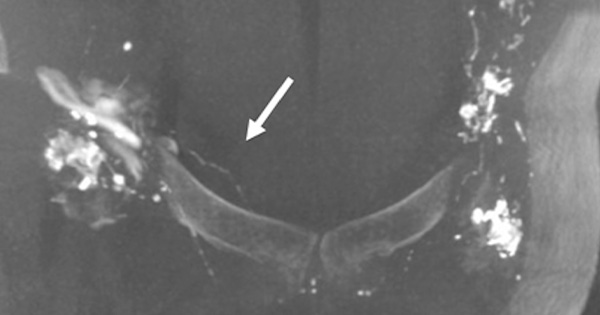
Patients with primary lymphoedema demonstrated a higher proportion of lymphovenous shunting between the lymphatic and venous systems (8/17 versus 0/17 in the lymphatic leak group, p=0.003, Table 3), as well as a higher proportion of passage of contrast past midline to the contralateral lymphatics (8/17 versus 0/15 in the lymphatic leak group, p=0.003, Figure 1). In two lymphatic leak patients, passage of contrast past midline could not be evaluated, either due to missing images or due to incomplete imaging of the pelvis.
A lower proportion of primary lymphoedema patients demonstrated opacification of the central lymphatics to the level of the renal vein (5/17 versus 10/14 in the lymphatic leak group, p=0.03) and to the level of the thorax (0/17 versus 5/14 in the lymphatic leak group, p=0.01). Of note, two lymphatic leak patients were injected from the neck lymph nodes and not the groin lymph nodes, and one lymphatic leak patient had incomplete imaging of the pelvis; these patients were not included for evaluation for opacification of the central lymphatics.
Discussion
In a unique direct comparison of patients with and without clinically diagnosed primary lymphoedema, notable differences in lymphangiographic patterns were found, with a higher rate of lymphovenous shunting, passage of contrast past midline to the contralateral lymphatics, and delayed opacification of central lymphatics seen in primary lymphoedema patients. These findings help define a degree of specificity with these drainage patterns in patients with a diagnosis of lymphoedema.
According to IL studies (Kim et al, 2024), anatomic studies (Pflug and Calnan, 1971), peripheral lymphangiogram studies (Edwards and Kinmonth, 1969), and nuclear medicine studies (O’Mahony et al, 2010), lymphovenous shunting is seen in 0–3% of normal healthy limbs (Edwards and Kinmonth, 1969) versus in 80% of patients with primary lymphoedema (Gosset al, 2019) and 50% of patients with secondary lymphoedema (Bains et al, 2015).
The frequency of lymphovenous shunting in this lymphatic leak cohort (patients without lymphoedema) was 0%, consistent with findings in the literature, while the frequency of lymphovenous shunting in this primary lymphoedema cohort was 47%, which is lower than the 80% previously noted in the nuclear medicine lymphoscintigraphy literature for primary lymphoedema (Goss et al, 2019).
Although the exact percentage of lymphovenous shunting in lymphoedema patients in unclear, it is nevertheless higher than in the non-lymphoedema population, concordant with lymphoscintigraphy studies in lymphoedema patients that show a relatively increased frequency of communication between the lymphatic system and venous vasculature proximal to the thoracic duct (Stamp and Peters, 2012; Soares et al, 2016).
Our study supports prior data indicating a higher rate of passage of contrast past midline to the contralateral lymphatics in primary lymphoedema patients (Liu et al, 2009) compared to the non-lymphoedema population in this study. This may represent decompression into less-stenosed, lower-pressure contralateral lymphatic channels. Delayed transit through the central lymphatics occurred in the lymphoedema population at an increased frequency, which may be due to an overall conduction delay or an underlying structural anatomic abnormality in primary lymphoedema patients (Williams et al, 2000).
Although a lower proportion of primary lymphoedema patients demonstrated opacification of the central lymphatics to the level of the renal vein, 3 out of 14 patients in the lymphatic leak group failed to opacify the central lymphatics to this level. This may be biased by the site of the leak (below the level of the chest) or termination of the study when the leak was identified (Table 2), without focus on the completeness of the study into the chest. Of note, two of the injections were in the neck, precluding evaluation of opacification of the central lymphatics.
Overall, the findings in this study represent a single-institutional observational experience in a limited patient cohort. Additional limitations include the fact that the control cohort involved patients with lymphatic leak as opposed to completely healthy controls and that the lymphatic leak IL images were reviewed retrospectively. Furthermore, while these patterns of drainage are more prevalent in primary lymphoedema patients, specificity to only primary, versus secondary, lymphoedema patients cannot be made. Future studies would ideally incorporate a larger cohort size for both groups to assess the frequencies of our findings more robustly, including a secondary lymphoedema cohort for additional comparisons.
Conclusion
Our study uses a contemporary central IL approach to substantiate that lymphovenous shunting, passage of contrast past midline to the contralateral lymphatics, and delayed opacification of central lymphatics, have increased prevalence in primary lymphoedema. This may be on an anatomic basis due to the underlying lymphatic pathology of primary lymphoedema.
These findings have the potential to aid the accurate diagnosis of instances of primary lymphoedema and add to the understanding of some of the underlying pathophysiology on a macrovascular level. Documentation of flow patterns may have implications for future primary lymphoedema therapy, including lymph node transfer location, benefits of lymphovenous bypass (Garza and Chang, 2018), and prediction of future lymphatic abnormalities on the contralateral side.