The extensive use of silver-based products has led the release and accumulation of silver in river, soil and other environments (Kale et al, 2021), this makes it much more likely that bacteria will develop resistance to silver. The aim of this article is to undertake a scoping review to identify the present state of antimicrobial resistance (AMR) to silver, particularly in regard to wound care pathogens, and to discuss the implications of these findings in terms of current and future treatment options.
Silver in wound care-related healthcare
Infected wounds may require topical and/or systemic antimicrobial therapy (depending on the level of infection) (Wound Infection in Clinical Practice Working Group, 2008). However, the rise of multidrug-resistant bacteria has shifted the preference away from antibiotics towards topical antimicrobial agents, such as antiseptics to prevent and treat infections, including biofilms (Schultz et al, 2017).
The development and use of wound dressings that offer broad-spectrum antimicrobial activity has been significant with the use of several mechanisms of action being employed (Yousefian et al, 2023), including the use of silver (Khansa et al, 2019), iodine (Barreto et al, 2020), polyhexamethylene biguanide (PHMB) (Rippon et al, 2023), dialkylcarbamoyl chloride (DACC) (Totty et al, 2017; Rippon et al, 2021) or medical grade (Manuka) honey (Scepankova et al, 2021). Antimicrobial wound dressings are widely used in wound care for their broad-spectrum antimicrobial properties (Yousefian et al, 2023). Among these, silver has been a primary choice for over two decades due to its effectiveness in combating infections and preventing biofilm formation (Frei et al, 2023).
Different forms of silver in wound care
Silver can be used in wound care in several different forms, including silver nitrate (a silver salt), silver sulfadiazine (a cream containing silver nitrate combined with sulfadiazine) and silver nanoparticles (AgNPs) (including nanocrystalline silver). Several silver-impregnated wound dressings have also been developed that provide silver to the wound. The role of AgNPs as an antimicrobial has gained significant attention due to their unique physiochemical properties that arise from the nanoscale dimensions of these particles (Rybka et al, 2022; Jangid et al, 2024). The positively charged silver ion (Ag+) is the predominant form of silver that has antimicrobial activity (Lansdown, 2006; Percival et al, 2012), targeting microorganisms via several different modes of action (Summer et al, 2024). However, AgNPs can have direct antimicrobial activity by, for example, interacting with bacterial cell walls (Yin et al, 2020; Summer et al, 2024).
Development of silver resistance
Despite its widespread use, concerns have emerged regarding the long-term effects of environmental exposure and the potential development of bacterial resistance to silver (Ferdous and Nemmar, 2020). This development is worrisome, as indiscriminate use of silver compounds, similar to the overuse of antibiotics, could reduce their effectiveness in treating infections (McNeilly et al, 2021). AgNPs, while effective, accumulating evidence suggests that bacteria can show resistance to AgNPs (Kamat and Kumari, 2023; Li and Xu, 2024; Rodrigues et al, 2024).
Silver resistance associated with wound care
The overuse of silver (silver ions and nanoparticles) in commercially available healthcare products, including wound dressings, is a growing as a potential health concern due to the possible selection of tolerant or resistant bacteria, diverging from the once commonly held perception that bacteria could not develop resistance to silver (McNeilly et al, 2021), and silver resistance is an important issue for healthcare professionals (Hosny et al., 2019; McNeilly et al, 2021; Blackburn et al., 2023). Percival et al (2019) noted that there are only a limited number of studies documenting evidence of silver resistance in bacteria.
However, in a review of the emerging concern for silver resistance in microorganisms, McNeilly et al (2021) highlight that many important pathogenic bacteria show silver resistance (Gupta et al, 1999; Gunawan et al, 2013; Muller and Merrett, 2014; Panáček et al, 2018; Hosny et al, 2019; Valentin et al, 2020). Hosny et al (2019), in their study of silver resistance in clinical isolates from wounds and burns, note that their study “… is alarming regarding the spread of phenotypic silver-resistance … in species where they were not detectable before”. Blackburn et al (2023) recently reviewed the evidence on AMR linked to antimicrobial use and observed that there is limited evidence to indicate that topical antimicrobials, including those containing silver, significantly contribute to resistance development. However, despite the limited clinical evidence, silver resistance is a critical issue for healthcare professionals, and the potential threat of resistance is a continuing essential area for exploration.
Silver resistance has become an increasingly critical area of research [Figure 1]. Recent research highlights the emergence of silver-resistant bacteria (Norton and Finley, 2021), the findings of which have been seen in studies in clinical settings where clinical isolates have been shown to exhibit silver resistance [Table 1]. This resistance could significantly impact current wound care practices, and the effectiveness of silver-containing medical devices, including silver wound dressings. For instance, laboratory analyses have demonstrated that some silver-impregnated dressings are less effective against resistant strains, suggesting the need for careful monitoring and judicious use of silver-based treatments (Sütterlin et al, 2012).
The issue of silver resistance aligns with broader concerns about AMR. Pathogens such as E. coli, S. aureus, A. baumannii, and P. aeruginosa have shown varying levels of silver resistance [Table 1]. These microorganisms are included in a group of important pathogens that are collectively referred to as the ESKAPE pathogens (Rice, 2008; Santajit and Indrawattana, 2016) and leading contributors to hospital-acquired infections and drug resistance (Ayobami et al, 2022; Bereanu et al, 2024).
A little-understood aspect of AMR is how heavy metals can impact antibiotic resistance. There are concerns of the coexistence of antibiotic and metal resistance: observational studies show that antibiotic-resistant bacteria are found at elevated levels in locations contaminated with metals (Gullberg et al, 2014). Antibiotic and metal resistance co-selection is where exposure to one selective agent allows adaptation to a second selective agent, i.e., where one antimicrobial (e.g., silver) selects for a resistance mechanism for both itself, and another antimicrobial (e.g., one or more antibiotics) (Gillieatt and Coleman, 2024). Three mechanisms have been suggested: co-resistance, where genes for resistance to different agents (e.g., silver and antibiotics) are on the same genetic element such as a plasmid, cross-resistance, where a single resistance mechanism (e.g., an efflux pump) provides resistance to multiple agents, and co-regulation, where the expression of multiple resistance genes is controlled by a shared regulatory system leading to simultaneous activation of resistance to different agents.
Implications of silver resistance in wound care
Given these developments, there is an urgent need to identify and utilize antimicrobial agents that are effective, non-toxic, and do not promote resistance. For example, DACC, a relatively new and alternate antimicrobial treatment option in wound care, shows promise in meeting these criteria (Totty et al, 2017; Rippon et al, 2021; Rippon et al, 2023a). Unlike silver, which acts via chemical mechanisms, DACC works by physically disrupting microbial adhesion, reducing the risk of resistance, and promoting healing without cytotoxic or adverse effects. Such alternatives could play a crucial role in addressing the challenges posed by AMR.
Methods
A preliminary search of the literature in PubMed using the search strategy ““silver” and resistance”” was conducted to assess the extent of the issue of silver resistance. A total of 9,531 references were identified from Jan-1975 (the first encountered silver-resistance was detected during an outbreak on a burns ward in 1975 (McHugh et al, 1975)) to end of 2023, with a further 852 articles being published between Jan-2024 and Oct-2024. Additionally, adding keywords “bacteria” and “microorganism” to the search strategy (to improve the relevance of the search) identified 5,502 references published in PubMed related to silver resistance in microorganisms, Note that, since 2020, there have been over 500 articles published per year [Figure 1] with, to Oct-2024, a further 514 articles have been published so far.
This scoping review was conducted in a semi-formal process and included several stages; (1) defining the research question; (2) identifying relevant studies; (3) study selection; (4) summarising and reporting the results. The research question was defined using the PEO framework [Table 2], which we considered an appropriate framework for our review (Doody and Bailey, 2016). The PEO framework breaks the topic of our review into three separate areas: the Population to focus on in the review, the Exposure (the issue of interest), and the Outcomes or themes to examine. Once the scope of the review was defined (to review the evidence for the development of silver resistance in microorganisms) relevant studies were identified. The PRISMA-ScR framework was used for reporting the results [Figure 2].
Research questions
Several research questions were identified as part of the development process for this review:
- Is silver resistance a rapidly developing issue in microorganism populations?
- Is silver resistance a rapidly developing issue in wound pathogens?
- What are the implications of silver resistance for wound care?
Search strategy and eligibility criteria
PubMed was searched using keywords “silver,” “resistant,” “resistance,” “bacteria,” “bacterial,” and “antimicrobial.” Articles published between January 1975 and October 15, 2024 were identified. Inclusion criteria for the search included primary evidence studies reporting silver resistance, laboratory, and clinical studies, with full-text articles written in the English language. Exclusion criteria included review articles, and papers not published in the English language.
Study selection, data extraction, and analysis
The process for study selection is outlined in the PRISMA-ScR flow diagram [Figure 2]. The titles and abstracts of each result was screened against the inclusion and exclusion criteria. Full texts of articles meeting the inclusion criteria were independently assessed by two authors (MR and AR). Following full-text screening, studies that met the inclusion criteria underwent data extraction. Collated information included the following data points: study aims and objectives; design and methodology; sample size; wound types; details of the outcome measures; and main study results [Table 3]. The primary outcome was the reporting of silver resistance in microorganisms.
Results
The findings were summarised and described narratively, under various collective headings based upon the research questions.
The search identified 5,295 potential records, and records from other sources (e.g., hand searching) (n=9) were added, resulting in 5,304 records. Following review of titles and abstracts, 105 full text articles were retrieved and included in the narrative review [Figure 2]. An overview of the study characteristics is presented in Table 4.
Note that many articles were identified in the initial review that were related to the use of “silver” against “drug-resistant” microorganisms (which did not involve any assessment of silver resistance); this led to them being captured by the search strategy. It was felt that adding any clarifying search keywords to try and reduce the number of these non-relevant articles may have impacted on capturing potentially relevant articles.
Discussion
Table 5 indicates the articles featuring ESKAPE pathogens (and other important microorganisms) reported to show phenotypic silver resistance.
Is silver resistance a rapidly developing issue in microorganism populations?
Considering the extensive use of silver ions as an antimicrobial in domestic, industrial, and medical applications (Ferdous and Nemmar, 2020), concerns have been raised over the potential for silver ion resistance emergence in bacteria of clinical relevance and to thereby compromise its therapeutic utility. Bacterial resistance to cationic silver (Ag+) has been recognized for several decades (Gupta et al., 1999), it was a common perception that resistance to nanosilver was unlikely, due to the multitargeting antimicrobial mechanisms of the nanoparticles (Gunawan et al, 2017; Valentin et al, 2020). There has been growing evidence regarding the development of adaptation phenomena to nanosilver, and this evidence has been observed in several bacterial species, including those of clinical significance, such as E. coli, P. aeruginosa, and S. aureus (Gunawan et al, 2013; Graves et al, 2015; Panáček et al, 2018; Valentin et al, 2020; Mann et al, 2021; Stabryla et al, 2021). The introduction of AgNP technologies and its effective use for treating wound infections have been suggested to reduce the likelihood of silver resistance (Pelgrift and Friedman, 2013). However, several articles have raised concerns over the development of silver resistance because of AgNP use (Kamat and Kumari, 2023; May et al, 2022).
The results from this review show that there is an increasing number of articles being published that are related to silver resistance in microorganisms, particularly in the last 5-10 years (n=105). Resistance to silver can be described as genotypic or phenotypic. Genotypic silver resistance refers to the inherent or acquired genetic mechanisms (determined by specific genes and plasmids) that allow bacteria to survive exposure to silver compounds (Randall et al, 2015). Phenotypic silver resistance refers to a microorganism’s observable ability to survive and grow in the presence of silver compounds, even though it doesn’t involve any genetic changes (Corona and Martinez, 2013). Phenotypic resistance include specific processes such as biofilm formation. Figure 3 shows the increase in articles in a subset of these 105 articles, namely, describing phenotypic silver resistance (n=50).
Wiegand et al (2012) analysed the adaptation capacity of S. aureus to several commonly used antiseptics (including silver nitrate). The bacteria were incubated with the appropriate IC50 concentrations of the antiseptics for 100 days. S. aureus quickly adapted to high concentrations of silver nitrate over time. The authors noted that, although silver-containing wound dressings were still as effective against silver-adapted S. aureus, overuse of silver may raise future problems. Elkrewi et al (2017) surveyed the prevalence of silver resistance in clinical isolates and reported that overt silver resistance is not common. However, upon silver challenge, high-level silver resistance was selected at high frequency in 76% of isolates of Enterobacter spp., 58% of isolates of Klebsiella spp., and 0.7% of isolates of E. coli. Other studies have shown that, although genes encoding silver resistance can be identified in a number of bacterial species, phenotypic resistance is relatively low. Silver resistance was examined in three clinically important Enterobacteriaceae genera (Sütterlin et al, 2017). Genes encoding silver resistance were detected most frequently in Enterobacter spp. (48%), followed by Klebsiella spp. (41%) and E. coli 4%. Phenotypical resistance to silver nitrate was found in Enterobacter (13%) and Klebsiella (3%) isolates. The results of Villapún et al (2021) suggest that the clinical use of silver is unlikely to select for silver resistance. Their studies used three bacteria from the World Health Organization (WHO) priority pathogens list: E. coli, P. aeruginosa and S. aureus. S. epidermidis was also studied. And the pathogens were evaluated against silver nitrate.
Several articles report the evolution of silver resistance of several microorganisms when challenged in vitro with AgNPs. McNeilly et al (2023) describe the development of a resistant phenotype in A. baumannii to AgNPs. Graves et al (2015), using a non-silver-resistant E. coli (using a strain that does not have any known silver resistance elements), found that that exposure to low concentrations of AgNPs resulted in the development of silver resistance, and that this resistance required relatively few mutation steps. A silver-sensitive P. aeruginosa, isolated from a burn wound and sensitive to AgNPs, exhibited reduced silver sensitivity with prolonged exposure to silver; a resistance that was reversed in the subsequent absence of silver (Mann et al, 2021). Stable acquisition of silver resistance has also been identified. Growing S. aureus in the presence of sublethal concentrations of AgNPs resulted in the retention of silver resistance in the microorganisms by multiple passages of the bacteria under silver-free conditions (Elbehiry et al, 2019).
The development of silver resistance in microorganisms is heterogeneous; not all microorganisms, when exposed to prolonged periods develop silver resistance. Mutations for silver resistance develop due to selection pressures exerted by silver-based antimicrobials, leading to the emergence of bacteria with mechanisms to tolerate or overcome silver’s toxicity. Bacteria mutation rates are high, typically ranging from 106 to 109 base substitutions per nucleotide per generation, but bacteria have been identified with approximately 100-fold higher mutation frequencies in clinical environments (Chevallereau et al, 2019). Natural genetic variation and the selective pressure exerted by prolonged exposure to silver results in heterogeneity in resistance (Habboush and Guzman, 2025).
Is silver resistance a rapidly developing issue in wound pathogens?
Antibiotics have been used widely for the treatment of wound infection. An alternate strategy to the use of antibiotics is the use of antimicrobial metals in the form of metal ions (e.g., silver ions) or nanoparticles. With the growing number of studies identifying the emergence of silver-resistant microorganisms, the implications for wounds and wound infection are particularly worrisome. Silver is considered one of the most potent, especially when prepared as nanoparticles (Roman et al, 2020). Silver has become commonplace in the clinical setting (Hussey et al, 2019). Despite this, examples of acquired resistance are rarely reported in the literature (Binsuwaidan et al, 2024).
Chronic wounds, however, provide a unique environment whereby silver dressings may be present in situ over prolonged periods of time, increasing the potential for bacterial phenotypic adaptation (Binsuwaidan et al, 2024). The use of silver-based treatments has especially increased in burn and wound care (Khansa et al, 2019). As a result of increased silver utilisation, questions concerning antimicrobial stewardship (AMS) and fears of widespread silver resistance emerging in clinical bacteria have been raised (Chopra, 2007). The use of AgNPs has been reported to result in the growth of resistant phenotypes (Gunawan et al, 2017; Panáček et al, 2018), thus calling into question the continuous and long-term utility of these nanomaterials in various formulations, wound dressings, medical devices, or other common household items. It is important to note that the WHO has developed a list of antibiotic resistant, global priority pathogens (WHO, 2024) as leading causes of nosocomial infections, collectively referred to as the ESKAPE pathogens (Rice, 2008; Santajit and Indrawattana, 2016). The ESKAPE organisms represent the model archetype of virulent and adaptive bacterial organisms, as they frequently cause severe and chronic disease and some of these pathogens are instrumental in wound infection.
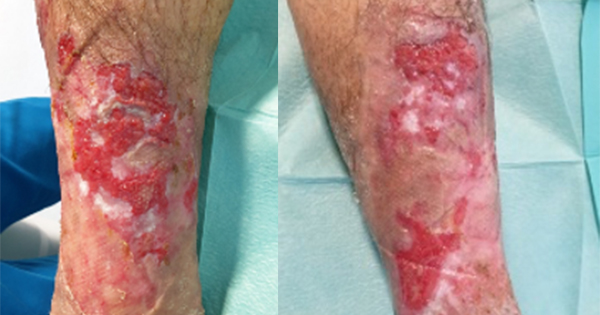
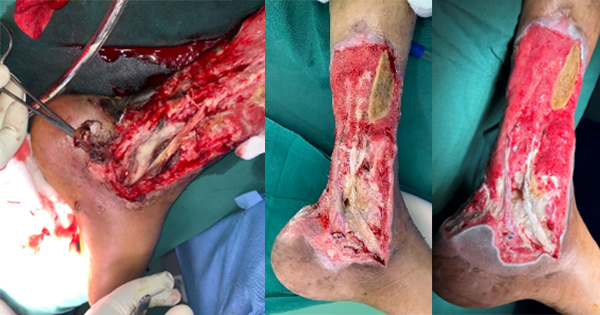
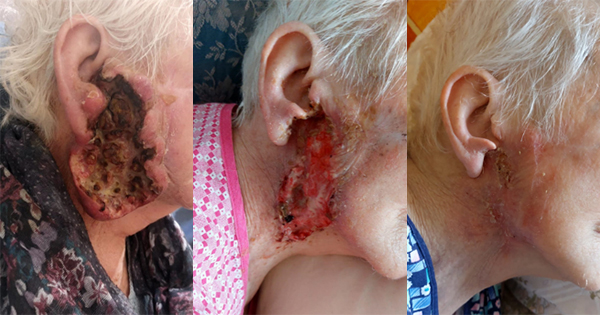
A number of articles have shown the presence of silver resistance in wound isolate microorganisms which reinforce the concern clinicians have that silver resistance in wound care is becoming a significant issue (Bridges et al, 1979; Pirnay et al, 2003; Loh et al, 2009; Woods et al, 2009; Sütterlin et al, 2012; Finley et al, 2015; Hosny et al, 2019; Percival et al, 2019; Safain et al, 2023; Binsuwaidan et al, 2024). Hosny et al (2019), examining 150 clinical isolates from burns and other wounds found 19 silver-resistant bacterial isolates. In a small study of 14 patients with chronic leg ulcers treated for 3 weeks with silver-based dressings, silver dressings had a limited effect on the primary pathogens (Sütterlin et al, 2012). In vitro evolution of silver resistance in these leg ulcer isolates indicated that it took only three weeks for silver resistance to emerge in isolates with silver resistance genes (sil genes). In a separate study, clinical isolates (E. coli, P. aeruginosa, S. aureus) isolated from patients with chronic diabetic foot wounds were assessed for their sensitivity to silver before and after passaging in the presence of ionic silver. Repeated exposure to ionic silver did not result in planktonic phenotypic silver resistance (Binsuwaidan et al, 2024).
However, there were significant changes in ulcer-derived Pseudomonas biofilm formation and sensitivity, with increased levels of biofilm formation being seen in this strain when cultured in the presence of silver. There was also an associated reduction in silver susceptibility.
Finley et al (2015) screened 859 clinical isolates (trauma and burn wounds) noted two isolates (K. pneumonia and E. cloacae) that were able to grow at high levels of silver, and estimated that only 0.2% had clinically significant phenotypic expression of silver resistance. However, they concluded that these results indicated silver resistance at a level that was capable of greatly reducing, if not negating, the effectiveness of most commercially available silver dressings, compared to non-resistant counterparts of the same species. The authors suggest silver resistance at a level that could significantly impact wound care and the use of silver-based dressings. Binsuwaidan et al (2024) highlighted that silver resistance in microorganisms is complex, particularly as there are no universally accepted guidelines to define phenotypic silver resistance.
Genetic (genotypic) and molecular biology (mechanism of action, MOA) analysis of silver resistance
Genomic-based silver resistance in bacteria is linked to genes and plasmids that code for silver resistance (Terzioğlu et al, 2022). Decreased susceptibility to silver ions/AgNPs has been linked to silver resistance genes encoding, for example, a silver binding protein, as well as additional genes involved in restricting the presence of silver within the microorganism (Maillard et al, 2021; Terzioğlu et al, 2022). Silver resistance can arise without the presence of silver resistance genes (Randall et al, 2015). Therefore, phenotypic silver resistance whereby microorganisms show signs of silver resistance is likely to provide a better indication of clinical impact than the presence or absence of silver resistance genes themselves. A recent review has proposed that the bacterial mechanism of silver resistance involves a combination of interconnected systems: (1) Inducing extracellular silver aggregation and reduction of Ag+ to Ag0; (2) Preventing silver from entering cells; (3) Efflux of silver in cells; (4) Self-repair of damage (Li and Xu, 2024).
Our review identified 55 articles related to the study of the genetics of silver resistance and/or the mechanism of action, particularly in the identification of genes and genetic elements in the genetic material of microorganisms that may confer silver resistance. Several microorganisms isolated from chronic diabetic foot wounds were identified as containing silver resistance genes (Percival et al, 2008). Two silver-resistant bacteria were identified: E. cloacae, a microorganism the authors point out that is rarely implicated as a primary pathogen in chronic wounds. They also find that no wound pathogens (S. aureus and P. aeruginosa) were found to contain silver-resistant genes. Studies assessing the prevalence of silver resistance genes animal and human wounds found that the presence of a silver-resistant gene did not afford protection to the organism in the presence of the silver dressing (Loh et al, 2009; Woods et al, 2009). Finley et al (2015) screened 859 clinical isolates (from trauma and burn wounds) and identified 31 that contained at least one silver resistance gene. However, despite having these genes most of the bacteria displayed little or no increase in resistance to ionic silver. Their findings suggest a low prevalence of these genes (3.6% minimum) occurring in hospital isolates and even fewer (0.2%) with clinically significant phenotypic expression of silver resistance. In a previously discussed study, Safain et al (2023) found that, although 65% (101/155) of sil gene-bearing isolates were resistant for silver nitrate, 17% (59/346) of the sil-negative isolates demonstrated phenotypic silver nitrate resistance (Safain et al, 2023).
What are the implications of silver resistance for wound care?
Finley et al (2015), assaying the effectiveness of silver-based wound dressings against silver resistant strains of microorganisms, indicated that silver resistance was at a level that is capable of greatly reducing, if not negating, the effectiveness of most commercially available silver dressings compared with non-resistant counterparts, and that acute emergence of silver resistance would have extensive consequences on wound therapies. As a consequence of the widespread development of microbial resistance to silver it is imperative to identify potential antimicrobial agents that may be used to combat wound infections that minimises or avoids the development of resistance, are not detrimental to healing (i.e., are not cytotoxic to tissues), do not cause adverse effects (e.g., allergic reactions), but have a wide range of antimicrobial activity. In addition, there should be strict monitoring on the use of silver in medical settings with the establishment of an approved standardised method for silver resistance detection (Hosny et al, 2019).
Our review identified several studies related to the development of therapies as alternatives to silver-based antimicrobials to circumvent the development of silver resistance. One study examined the potential of bismuth-based nanoparticles (BiNPs) (Pant et al, 2022). Another suggests that covalent bonding of silver to cyanographene over comes silver resistance to AgNPs (Panáček et al, 2021). Pant et al (2022) showed that BiNPs exhibited a potent broad-spectrum antimicrobial activity against P. aeruginosa and S. aureus, and were effective against silver resistant bacteria. BiNPs did not appear to contribute to the development of bismuth-resistant phenotypes after 30 passages of consecutive exposure to sub-lethal doses of NPs. Cyanographene is an efficient covalent trap for silver ions, and strong covalent immobilisation of silver and has potent antibacterial activity (similar to free silver ions), activity in AgNP-resistant bacterial strains, and very low leaching of silver ions or AgNPs (Panáček et al, 2021). A nanohybrid (AgNPs supported on 1nm-thick silicate “platelets”) showed a strong antibacterial activity on all pathogens assessed, including silver-resistant E. coli (Su et al, 2011). The authors suggest that the nanohybrid bypasses the usual ionic silver mechanisms on silver-resistant bacteria leading to effective antibacterial activity in silver-resistant E. coli.
Conclusion
This review has identified a substantial number of articles that provide evidence that antimicrobial silver resistance is a growing problem in healthcare. In the general healthcare landscape, a wide variety of pathogens have been identified that have developed silver resistance [Table 5]. The implications of this resistance are serious in that silver is widely used in healthcare, domestic and agricultural applications. Additionally, and importantly, silver treatments are widely used in the prevention/treatment of infection in wounds as dressings, irrigation/instillation solutions and gels etc., to great effect, with an initial two-weeks treatment recommendation followed by a management re-evaluation (Leaper, 2012). But several authors have described wound pathogens as developing resistance, and thus becoming difficult to treat when infection occurs.
Ultimately, the impact of this development of resistance is that these treatments become less effective and the toolbox available to clinicians diminishes. Therefore, while silver remains a valuable tool in wound care, its use should be cautious and accompanied by ongoing research into resistance mechanisms and alternative treatments. The development of new antimicrobial agents that rely upon chemical mechanisms (e.g., bismuth) may also result in the development of resistance. A focus a on physical mechanism-based approach to antimicrobial action may avoid chemical-based resistance mechanisms in the future.
Combining antimicrobial agents with a targeted and sustainable approach could help mitigate the risks associated with resistance and ensure effective wound management. Because of the widespread development of silver resistance, resistance monitoring should form part of AMS practices.
Also, it is imperative that alternative treatments to antibiotics and some antiseptics (e.g., silver) are identified and used as alternatives. These alternatives should have a good in vitro and in vivo/clinical evidence base, not cause any detriment to healing and be cost-effective. In addition, it is important to identify novel antimicrobial agents that do not cause resistance but, at the same time, have wide ranging antimicrobial activity. DACC, which acts via a physical-based mechanism which does not lead to the development of resistance, is an example of an antimicrobial agent used in wound dressings that is today providing a route to delivering effective antimicrobial activity with no resistance.