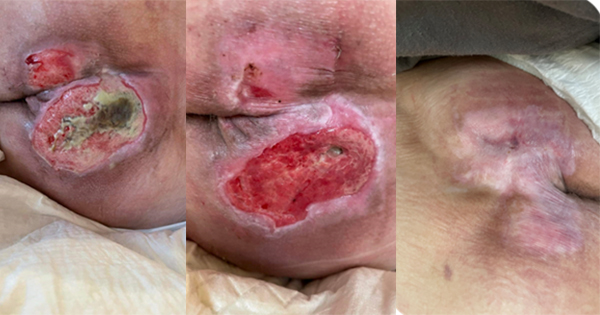
<p>This paper, written by an international group of experts in the bioengineering and clinical aspects of the design, use and evaluation of dressings for pressure ulcer prevention, addresses a central question commonly faced by the medical device industry, clinicians and patients. The question being whether evidence obtained for a specific product can be extrapolated to other products, which are similar or lookalikes, and are made by different manufacturers. Specifically, this question is of fundamental importance to wound care clinicians and particularly in the area of dressings used in the prophylaxis of pressure ulcers (also called pressure injuries in the US and Australia). The authors thoroughly discuss recent developments and litigation in the medical device industry, relevant regulation routes in the pharmaceutical industry aimed at ensuring patient safety, and examples from the automotive industry to describe the great danger in extrapolating bioengineering and medical evidence obtained for one dressing product to other products by different manufacturers. The contents of this paper demonstrate why the question clinicians must ask before selecting a prophylactic dressing is: “Will I choose a dressing based on marketing hype and cost or, alternatively, based on published scientific, bioengineering and ultimately clinical evidence?” </p>