Session one – Health economics in wound management: Pinaki Ghosh, B. Braun Medical Industries, Pulau Pinang, Malaysia
Pinaki Ghosh began the session by discussing health economics in wound management. An overview of the general ideal of health economic data was shared, including the importance of taking a value-based healthcare (VBH) approach to achieve better outcomes for patients and deliver cost efficiencies for healthcare systems. The concept of VBH was first proposed in the mid-1990s by Michael Porter and Elizabeth Teisberg (Porter and Teisberg, 2006; Teisberg et al, 2020). VBH is an approach that aims to focus decision-making on achieving the best outcomes for patients with the resources that are available, either by producing better outcomes with the same resources or by producing the same outcomes at a lower cost (Posnett, 2022).
There is a lack of consistency in the definition of VBH in the literature; however, VBH has been defined as “the equitable, sustainable and transparent use of the available resources to achieve better outcomes and experiences for every person” (Hurst et al, 2019). Furthermore, Pinaki emphasised that ‘value’ is derived from measuring health outcomes against the cost of delivering the outcomes [Figure 1].
The use of cost-effectiveness analysis as a tool to guide resource allocation has revolved around the Incremental Cost Effectiveness Ratio (ICER; Box 1; Hounton and Newlands, 2012). By plotting the incremental effectiveness of a treatment (relative to a comparator) against the incremental cost of treatment, cost-effectiveness can be represented visually where effectiveness and total health benefits increases from left to right on the x-axis, and total cost increases with the rise of the y-axis. In the case of most treatments, there is a need of a threshold value that the decision maker is willing to pay as a good value for money (Hounton and Newlands, 2012).
VBH has benefits for patients (e.g. reduced infections, time to wound closure, odour and pain, and improved quality of life and wound bed preparation), clinicians (e.g. reduced nurse time and visits, released clinical hours for treating more patients, better resource utilisation and allocation, and reduced hospitalisations) and healthcare systems (e.g. reduced burden, allowing them to form better policies and cater to the unmet needs of patients). The ultimate goal of VBH is to reduce infections/complications, medication costs and prolonged hospital stays, and create cost efficiencies for healthcare systems and better quality of life for patients.
Second session — Results of the single-blind RCT, ‘Effects of a wound cleansing solution on wound bed preparation and inflammation in chronic wounds’: Andrea Bellingeri, Head Nurse, Wound Care Ambulatory Service Foundation, San Matteo, Pavia, Italy
The second session opened with Andrea Bellingeri and focused on the use of surfactants for wound bed preparation in chronic wounds.
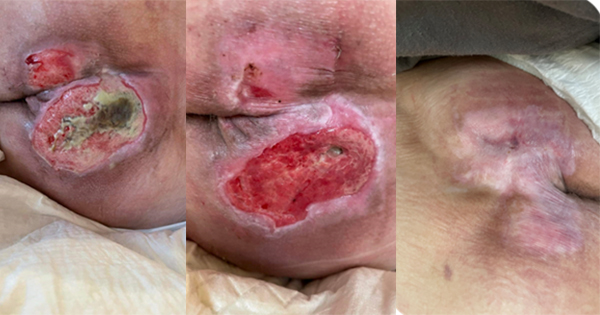
A study by Bellingeri et al (2016) on ‘Effects of a wound cleansing solution on wound bed preparation and inflammation in chronic wounds: a single-blind RCT’ assessed the clinical efficacy of a propylbetaine-polihexanide (PP) solution (Prontosan® Wound Irrigation Solution) versus normal saline (NS) solution in wound bed preparation on 289 patients.
Prontosan Wound Irrigation Solution was used, which contains betaine surfactant and polyhexanide, and has been indicated for the removal of biofilm in acute, chronic and burn wounds. During the investigation, males and females aged >18 years were recruited that complied with the following parameters: (1) presence of at least one pressure ulcer stage 2 or 3 or the presence of a partial thickness lesion of vascular origin and (2) wounds treated with advanced dressings — e.g. polyurethane, alginate, hydrocolloid, hydrogel and non-adherent contact layers.
Outcomes were measured using the Bates-Jensen Wound Assessment Tool (BWAT; 13 items, score 12–60). Measures included wound characteristics such as inflammation, peripheral tissue, oedema and induration, exudate amount, and type and amount of necrotic tissue. Importantly, evaluation of wound evolution and inflammatory signs took place at inclusion (T0), day 7 (T1), day 2 (T3) and day 28 (T4), and outcomes were comparatively analysed using two-tailed Student’s t-tests.
Both groups had similar demographics, clinical status and wound characteristics, and statistically significant differences were seen between T0 and T4 for the following outcomes: BWAT total score, P=0.0248; BWAT score for inflammatory items, P=0.03; BWAT scores for wound size reduction (P=0.049) and granulation tissue improvement (P=0.043), all in favour of PP. Visual Analog Score for pain was similar in both groups and there were no adverse events related to the treatment during the study period.
The results of the study show that compared to saline solution, Prontosan Wound Irrigation Solution promotes wound bed preparation, reduces inflammatory signs and accelerates healing in venous leg ulcers and pressure ulcers. Overall, the key take-home message from the session was that more research is needed on the use of antiseptics and cleansing solutions for chronic wounds.
Pinaki then went on to discuss the use of clinical evidence to quantify cost-effectiveness, as well as the concept of dimensions of value in wound care [Box 2].
The concept of a wound condition model was then explored, including the use of linear regression in studies investigating wound bed preparation. For example, linear regression has been used to estimate time to wound bed preparation (NICE, 2022). The time-dependent improvement of the wound bed (BWAT score) was captured by formulating an equation for the wound improvement as a function of time and fitting the constants in the equation to observed data.
Vidal (2016) showed that “continuous linear healing rate was more accurate with higher values and requires less quantifications than usual formulas to make a wound-healing projection”. It was also emphasised that “observed linear healing rates are approximately constant over time and that they should be able to predict a time to total healing for chronic wounds” (Gilman, 2004). Moreover, Robson et al (2000) used linear regression analysis to predict when all patients would achieve 100% closure. The findings of Bellingeri et al (2016) demonstrating comparative BWAT scores of Prontosan and saline over the period of wound bed preparation was cited. It was demonstrated how linear regression was used to project the comparative average estimated time to achieve wound bed preparation using Prontosan (Average 28.90 95% CI [24.21, 37.24] days) and sterile saline (Average 78.93 95% CI [58.68, 130.6] days) respectively, considering BWAT score of 14 as composite endpoint reflecting various dimensions of wound bed preparation. Additionally, the formula to compute price of therapy which enables determination of costs and benefits based on the wound condition model was proposed and is shown in Figure 2.
Pinaki also presented published results of the above wound condition model (Suh and Ghosh 2020) which reported that total costs with 0.1% polyhexamethylene biguanide and betaine was 63.6% lower compared to sterile saline, over the complete wound healing process. Moreover, the cost incurred due to antibiotics (including the costs of IV/oral antibiotic therapy) in 0.1% polyhexamethylene biguanide and betaine patients was 83.3% lower compared to sterile saline.
Pinaki then went on to describe the wound closure model and introduced the concept of a monthly probability of infection and wound healing. Pinaki cited the study by Andriessen and Eberlein (2008), reporting data on comparative healing and infection outcomes of 0.1% polyhexamethylene biguanide and sterile saline. A predictive model based on this study was developed which used monthly probabilities over 6 months and calculated the monthly healing and infection probabilities over a period of 12 months for 0.1% polyhexamethylene biguanide and sterile, respectively. Pinaki then discussed a study by Cooper et al (2023) in which the researchers undertook a cost-effective analysis to investigate the use of Prontosan Wound Irrigation Solution as compared to the standard practice of using saline for treating venous leg ulcers (VLUs). Cooper and colleagues utilised a Markov model that was parameterised to one-year costs and quality of life (QoL) consequences of venous leg ulcers (VLU) treatment with Prontosan versus saline.
A systematic literature search was conducted to inform the clinical parameters of the economic model. Primary effectiveness measure was quality-adjusted life years (QALYs) gain per patient, which was reported as open ulcer 0.75, infected ulcer 0.70 and healed ulcer 0.84. Cost parameters for assessing cost-effectiveness of Prontosan Wound Irrigation Solution versus saline were also reported as healed wound £33.96, open wound £507.84 and infected wound £1,898.54.
Monthly costs of treating open and infected wounds with Prontosan Wound Irrigation Solution was £29.53 and £36.47, respectively. In the saline group, treatment costs for open wounds was £2.69 and for infected wounds, it was £3.33. However, since Prontosan-treated wounds spent less time in the open and infected wound states, and longer time in the healed state (compared with saline group), it was emphasised that movement of patients across health states will drive the differences in cost between the two treatment groups. Overall, results showed that use of Prontosan Wound Irrigation Solution is cost-effective and provides a net cost saving of £867.87 per patient over a 1-year period and 0.0087 QALYs per patient.
Third session – Cost-effectiveness in clinical practice: improving our management of hard-to-heal wounds: Alison Vallejo, University of the Sunshine Coast, Australia
Alison Vallejo opened the third session by discussing the burden of chronic wounds in Australia and issues related to global antimicrobial resistance (AMR) and, therefore, the importance of cost-effectiveness in clinical practice. It was emphasised that chronic wounds are different to acute wounds — they are a challenge to treat and sometimes fail to heal altogether. It was stressed that chronic wounds affect around 420,000 Australians (AusHSI, 2017; Wounds Australia, 2022). The cost of wound care products is expected to reach US $15–22 billion annually (Sen, 2019), and this rise is associated with an ageing population and increasing prevalence of comorbidities — e.g. diabetes and obesity.
Vallejo then went on to explain what clinicians can do to help improve the management of hard-to-heal wounds and outcomes for patients, including:
- Change our practices and play our part in promoting antimicrobial stewardship (AMS)
- Provide our patients with the best possible care, by using more appropriate products and re-examine our practices
- Be aware of the evidence and stay up-to-date by practicing evidence-based wound care
- Remember our obligation to base our day-to-day practice on the evidence (Wounds Australia, 2016), and promote better wound hygiene practices in the most cost-effective way.
Next, Vallejo encouraged clinicians to re-examine their practice and she provided the following reminders (Hotaling and Black, 2021; IWII, 2022):
- Pressure, fluid and chemicals are needed to remove microscopic biofilm and other debris from the wound surface
- Normal saline has previously been the most common solution for cleansing wounds due to its lack of harm to the tissue
- Saline contains no properties to reduce bioburden, and vials used do not have any pressure and are unsterile once opened
- The use of aggressive antiseptics like hydrogen peroxide destroys all tissues
- The use of advanced topical antiseptics and surfactants in prepared bottles for correct pressure application is safe on healthy tissue and effective on harmful bacteria.
Vallejo then went on to discuss her experiences and research. A study by Vallejo et al (2021) on the ‘Use of low-frequency contact ultrasonic debridement (LFCUD) with and without polyhexamethylene biguanide in hard-to-heal ulcers: an RCT’ aimed to investigate the effect of Prontosan Wound Irrigation Solution with LFCUD compared to LFCUD alone in patients with hard-to-heal wounds. The single-blinded randomised controlled trial recruited adults aged 18 and over who had a chronic wound for over 6 weeks that was assessed as being suitable for LFCUD. Exclusion criteria included cognitive impairment, malignancy, spreading infection, autoimmune disease, diabetes, sensitivity to 0.1% polyhexamethylene biguanide and betaine, pregnancy, exposed cartilage and wounds <2cm2 or >100cm2. Primary and secondary outcomes of the investigation are shown in Box 3.
During the first 6 weeks, the intervention group were treated weekly with LFCUD, and Prontosan Wound Irrigation Solution soak for approximately 15 minutes. Participants’ wounds were covered with a Prontosan Wound Irrigation Solution sustained impregnated dressing and were given concurrent care — i.e. compression therapy for 12 weeks. In the control group, participants were treated weekly with LFCUD, but their wounds were washed with clean water or saline wash only. Their wounds were dressed with a non-medicated dressing, and they received the same concurrent care as the intervention group.
Vallejo and colleagues found that in the control group, 44% of wounds deteriorated compared to 4% in the intervention group (P=0.003). It was also found that CFUs steadily increased in the control group and reduced in the Prontosan Wound Irrigation Solution group (P=0.01). Eighty percent of the wounds in the intervention group were followed to the end at 12 weeks, compared to 52% in the control (P=0.03), and wound size reduction rate was 61% in the Prontosan Wound Irrigation Solution group compared to 12.7% in the control (P=0.019). Overall, pain was reduced significantly at week 6 in the Prontosan Wound Irrigation Solution group (P=0.04). The key take-away message of Vallejo’s session was that there is increasing evidence to show Prontosan Wound Irrigation Solution and betaine as important tools in the fight against biofilm. Furthermore, benefits of Prontosan Wound Irrigation Solution that were described included:
- Improves outcomes when debridement is used
- Prevents wound infection and reduces pain
- Reduces the need for antibiotics, hospital admission and assists with AMS
- Has no acquired resistance to date, which has been said to be unimaginable (Kramer et al, 2019).
However, it was stressed that more research is needed. Vallejo discussed the need for well-designed health economic studies to confirm the real-world cost-effectiveness, and the session was concluded with a brief overview on the future of health economic data.