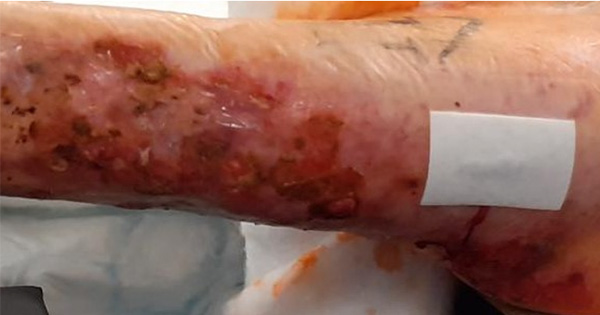
<p>Incontinence-associated dermatitis: why do we need a core outcome set for clinical research?Incontinence-associated dermatitis (IAD) is a type of irritant contact dermatitis related to chemical and physical irritation of the skin barrier, triggering inflammation and subsequent skin damage. Management of IAD should essentially focus on skin cleansing to remove the irritant, debris and microorganisms; skin moisturisation to repair or augment the skin’s barrier, retain and/or increase its water content, reduce transepidermal water loss and restore or improve the intercellular lipid structure; and the application of a skin barrier product to prevent skin breakdown by providing an impermeable or semi-permeable barrier on the skin. The lack of comparability between studies about efficacy and (cost-)effectiveness of products and procedures complicates standardisation of IAD management. To overcome this challenge, the development and use of a Core Outcome Set (COS) is needed. A COS is a consensus-derived minimum set of outcomes that should be measured and reported in clinical trials of a specific health condition. A 2018 international study at Ghent University concluded that erythema, erosion, maceration, IAD-related pain and patient satisfaction are core outcome domains in IAD clinical research. Identical outcomes across trials will allow comparability of results and, thus, enhance the value of evidence synthesis and reduce the risk of outcome reporting bias. </p>