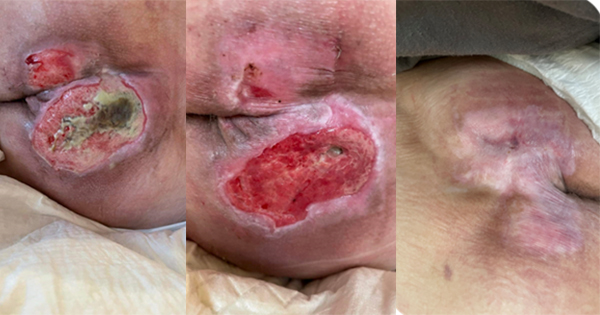
In 2010, the automobile manufacturer, Toyota, had received over 100 complaints about brake problems. When the brakes failed on a car in California and lead to the death of four people, Toyota recalled over 2.5 million cars to have them inspected and repaired. Over the next 4 months, the company recalled 3.4 million more vehicles in three separate recalls, resulting in a total of just under 7 million. There were several issues: potentially sticky gas pedals, pedal entrapment and software glitches that affected braking on some models. You might ask: “What does this have to do with medical device-related pressure ulcers (MDRPUs)?” When you consider the reason Toyota recalled the cars — to prevent future harm.