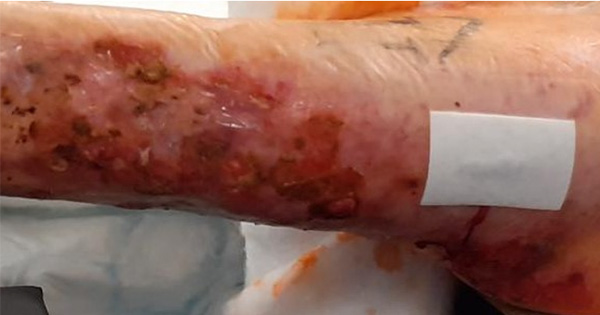
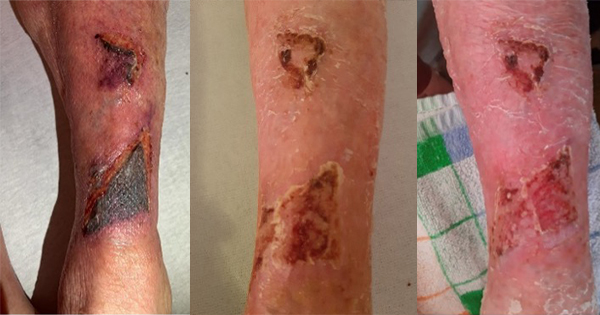
<p>The aim of this study was to assess the ability of an absorbent foam dressing in preventing post-operative wound blistering following hip and knee arthroplasty. Complications following hip and knee arthroplasty are surprisingly common with widespread reports of wound blistering, leakage and infection. The selection of suitable dressings for the treatment of such wounds is, therefore, an important part of surgical wound care management. Until 2001, a traditional absorbent dressing (Cosmopor® E; Hartmann) was used at Alingsas Hospital, in Sweden, for the management of surgical wounds but persistent leakage resulted in the need for frequent dressing changes, increasing the risk of infection. Wound blistering was also a problem. The introduction of an Aquacel® (ConvaTec)/Tegaderm™ (3M Health Care) dressing combination did address absorption concerns but wound blistering remained an issue. Mepilex® Border (Mölnlycke Health Care), an absorbent foam dressing incorporating Safetac® (soft silicone) technology, was subsequently introduced with the ultimate aim of preventing periwound skin blister formation during the post-operative treatment period following hip and knee arthroplasty. The absorbent foam dressing was applied to 146 patients who had undergone scheduled hip or knee arthroplasty. Dressings were changed on the fourth postoperative day or earlier if there was a clinical need. The post-operative wound status and dressing performance were recorded.</p>