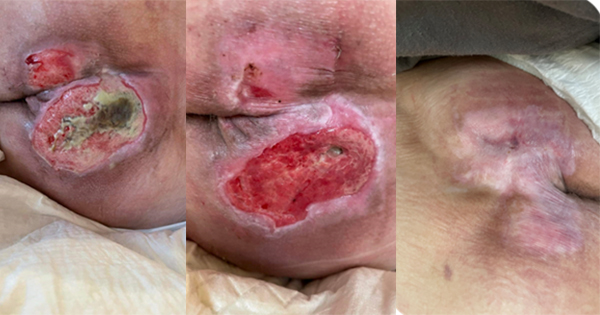
This article reviews the innovative SEM Scanner (Bruin Biometrics) technology for early detection of a forming pressure ulcer (PU) from a 360-degree perspective, considering the physiological, biophysical, medical-clinical and cost-effectiveness points of view, altogether. The SEM Scanner is designed to help healthcare professionals address a major medical need: pressure ulcer prevention (PUP). Currently, there is no technology other than the SEM Scanner for supporting clinicians in their decision-making with regards to the PU risk at specific anatomical sites of their patients. Based upon wellestablished physiological and biophysical principles underlying the aetiology of PUs, the SEM Scanner is targeting a specific stage in the PU cascade, in which there is a window of opportunity for detection of a localised change in the biocapacitance property of a tissue region at risk. Such change would indicate inflammatory micro-damage that may still be reversible. This is in stark contrast with the conventional clinical thinking of documenting an already existing structural tissue damage, which occurs much later in the injury spiral, typically days after the onset of the micro-damage. In other words, only when the damage becomes macroscopic and wide-spread, it can be spotted by the traditional visual skin assessment (VSA) practice or by an ultrasound examination. The benefits of a quantitative, standardised and objective early-detection of PUs, using the SEM Scanner as an adjunct to the currently subjective process of PU identification, make this device a disruptive innovation, particularly considering that the risks in using this device, if any, are negligible. The SEM Scanner technology has both proven clinical successfulness and cost-effectiveness. Risk assessment and early-detection are the two essential foundations for effective PUP.