Diabetes is a leading cause of death and disability worldwide. Just under half a billion people are living with diabetes worldwide, and this is projected to increase by 25% in 2030 and by 51% in 2045 (Saeedi et al, 2019).
Over the period 1999–2017, non-communicable diseases were the leading cause of death in Aruba. The top four causes of death were cardiovascular diseases, cancers, diabetes and chronic respiratory diseases (Pan American Health Organization, 2023). Aruba has one of the highest percentages of diabetes in the world – of the 112,000 citizens of Aruba, 16.24% have diabetes, versus 8.33% globally (Aruba Today, 2019).
The prevalence of DPN ranges from 7% within 1 year of diagnosis to 50% for those with diabetes for >25 years. It is often the first complication associated with diabetes, as well as the most common complication of the disease. Distal sensory nerve fibre degeneration, axonal loss, and microangiopathy of the endoneurium are characteristic findings in DPN (Yagihashi et al, 2011).
The most common clinical presentation of DPN is distal symmetrical decreased sensation to the lower extremities varying from mildly decreased sensation to complete loss of sensation. Patients often describe feeling as though they are wearing an invisible glove or stocking.
About 20% of people with DPN may also develop neuropathic pain (Gylfadottir et al, 2019). Symptoms may include a burning sensation or freezing pain, sharp jabbing shooting electric-like pain, extreme sensitivity to touch, and loss of balance and coordination. Patients with diabetes often have poor sleep habits, including difficulty falling asleep or staying asleep, and DPN is another cause of sleep disruption (Kaur et al, 2011). Symptoms most commonly start in the great toes and ascend bilaterally and symmetrically in a length-dependent manner over time. Eventually, symptoms may affect the hands. Loss of protective sensation due to DPN is a leading cause of lower-extremity amputations (National Institute of Diabetes and Digestive and Kidney Diseases, 2018).
During episodes of hyperglycaemia in diabetes, most cells keep their internal levels of glucose constant. This is not the case with the capillary endothelial cells in the retina, mesangial cells in the renal glomerulus, and neurons and Schwann cells of the peripheral nervous system. These cell types undergo increased levels of intracellular glucose during episodes of hyperglycaemia. The rise of internal glucose levels in these cell types activates multiple pathways that cause complications, including the polyol pathway, advanced glycation end products pathway, protein kinase C pathway, and the hexosamine pathway. Activation of these pathways damages these cells via increased oxidative stress due to the overproduction of superoxide radicals by the mitochondrial electron transport chain (Brownlee, 2005). The damage to these cell types correlates with common complications of the disease including blindness, kidney disease, vascular disease, and, as it pertains to this discussion, peripheral neuropathy.
Thiamine, also known as vitamin B1, is an essential nutrient needed for many key biochemical and physiological processes. Thiamine is essential for Krebs cycle activity and, as such, for proper intracellular energy production in the form of adenosine triphosphate via mitochondrial oxidative phosphorylation. Thiamine deficiency reduces adenosine triphosphate production and has emerged as a likely mechanism in neurodegeneration (Jadiya et al, 2021).
Thiamine deficiency has been shown to induce oxidative stress, endoplasmic reticulum stress, and autophagy in the brain. These processes are known to contribute to the pathogenesis of various neurodegenerative diseases, including peripheral neuropathy (Liu et al, 2017). There appears to be a correlation between diabetes, a disease of impaired carbohydrate metabolism, and thiamine deficiency, a cause of impaired carbohydrate metabolism (Beltramo et al, 2008).
Plasma levels of thiamine in patients with diabetes were 75% less when compared to levels in a healthy population (Rabbani and Thornalley, 2018). Thiamine is synthesised exclusively by bacteria, fungi and plants (Vistoli et al, 2013). Humans do not manufacture thiamine and must obtain it from their diet. Thiamine is water soluble and absorbed from the gut via the action of thiamine transporter molecules (Mrowicka et al, 2023).
Benfotiamine (S-benzoyl-O-monophosphate) is a lipid soluble thiamine derivative belonging to the class of allithiamines (Bozic and Lavrnja, 2023). It is absorbed from the gut via passive diffusion and increases plasma thiamine levels to a much greater extent than the water-soluble form. Benfotiamine has been shown to inhibit the complication-causing pathways associated with diabetic pathobiology, including the polyol pathway, advanced glycation end products pathway, protein kinase C pathway, and the hexosamine pathway (Hammes et al, 2003; Berrone et al, 2006).
Benfotiamine has been shown to improve the symptoms of DPN (Stracke et al, 2008). Vitamin B12 deficiency, a well-established cause of polyneuropathy, is common in type 2 diabetes patients, especially those taking metformin (Pflipsen et al, 2009).
Methods
A retrospective, qualitative analysis was conducted on three patients (n=3) with DPN at the wound care centre of the Dr Horacio E Oduber Hospital in Oranjestad, Aruba. Each patient underwent weekly clinical visits for assessment and treatment.
Inclusion criteria
Patients with symptoms of polyneuropathy secondary to diabetes, such as pain, numbness, burning and/or tingling in the legs and feet.
Exclusion criteria
1) Hypersensitivity to benfotiamine or thiamine. 2) Patients who were non-adherent with the instructions for use. 3) Pregnant or lactating women.
Instructions for use
The patients were given a proprietary formulation of benfotiamine 300 mg and methylcobalamin 1 mg capsules twice daily with food for 30 days (loading dose) and then once a day thereafter (maintenance dose). The patients were scheduled to return to the clinic weekly for follow-up evaluation.
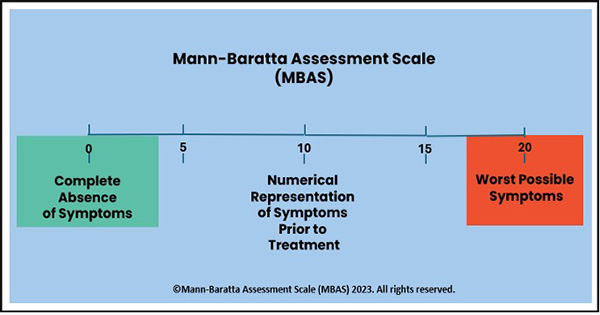
Mann-Baratta Assessment Scale
The Mann-Baratta Assessment Scale (MBAS) was designed by Dr Mann and Dr. Baratta to measure a patient’s perceived changes in symptoms during therapy. In this case, it was used to evaluate subjectively perceived changes in neuropathic symptoms.
The patient was asked the following questions at each weekly clinical visit: “If we assign a numerical value of 10 to the neuropathy symptoms (pain, numbness, burning, tingling, etc.) that you felt in your feet and legs before starting this trial (before taking the proprietary formulation) and we assign the numerical value of zero (0) to a complete remission of the symptoms and a value of 20 to the worst possible symptoms, what numerical value would you give your symptoms today? Remember, ‘10’ is what you started with, ‘0’ is complete remission, and ’20’ is what you would feel if you had the worst possible symptoms.” The patient’s numerical response was recorded.
Ethics committee approval
The study was approved by the ethics committee of the Dr Horacio E Oduber Hospital Oranjestad, Aruba.
Case reports
Patient 1: A 68-year-old male construction worker presented to the clinic for treatment of a non-healing venous ulcer on the right leg of 5 years’ duration. The patient presented with chronic numbness and tingling in both lower extremities. The patient was diagnosed with type 2 diabetes 10 years ago and DPN 8 years ago. He had sought previous consultation with a physician for DPN, but was not prescribed any medication.
He was started on a proprietary formulation of benfotiamine 300 mg and methylcobalamin 1 mg capsules twice daily with food for 30 days (loading dose) and then once a day thereafter (maintenance dose). Following initiation of this therapy, the patient’s symptoms persisted unchanged in weeks 1 and 2 (MBAS = −10). On week 3, the patient reported that his symptoms were completely relieved (MBAS = 0). The patient was managed and monitored for 17 weeks, and over the course of that time, the patient reported continued relief of his symptoms (MBAS = 0; Figure 2).
Patient 2: A 65-year-old woman presented to the clinic for treatment of a non-healing venous ulceration to the left leg of 5 years’ duration. The patient presented with chronic numbness, tingling and shooting pains in both lower extremities. She was diagnosed with type 2 diabetes 15 years ago, with DPN diagnosed 10 years ago. She was started on a proprietary formulation of benfotiamine 300 mg and methylcobalamin 1 mg capsules twice daily with food for 30 days (loading dose) and then once a day thereafter (maintenance dose). Following initiation of this therapy, the patient’s symptoms persisted unchanged in weeks 1 and 2 (MBAS = −10). On week 3, the patient reported that her symptoms were completely relieved (MBAS = 0). The patient was managed and monitored for 12 weeks, and over the course of that time, the patient reported continued relief of her symptoms (MBAS = 0; Figure 3).
Patient 3: A 52-year-old woman presented to the clinic for treatment of a venous stasis wound, unrelenting burning pain, numbness and tingling to her lower extremities. The patient was diagnosed with type 2 diabetes 5 years ago, with DPN was diagnosed 3 years ago. She was started on a proprietary formulation of benfotiamine 300 mg and methylcobalamin 1 mg capsules twice daily with food for 30 days (loading dose) and then once a day thereafter (maintenance dose). Following initiation of this therapy, she had decreased numbness, tingling and burning pain (MBAS = 6) in week 2. In week 3, she reported continued improvement, primarily with her burning pain (MBAS = 4). In week 4, the patient had complete relief of her symptoms (MBAS = 0), which continued through weeks 5 and 6 (MBAS = 0; Figure 4).
Discussion
An association between a deficiency of vitamin B1 and degenerative neurological disorders is apparent, as substantiated by existing literature. This is true of vitamin B12 deficiency as well. The three patients reported in the paper exhibited characteristic symptoms of DPN. All three patients reported significant improvement in their symptoms after the administration of a proprietary formulation comprising benfotiamine 300 mg and methylcobalamin 1 mg capsules over a 30-day loading dose period. Notably, all three patients reported an absence of symptoms, as indicated by a MBAS of 0, within 4 weeks.
Conclusion
There is an apparent role for the combination of benfotiamine and methylcobalamin in managing peripheral neuropathy.
After 3 or 4 weeks of therapy, all patients experienced a complete relief of their symptoms, as indicated by a MBAS = 0. Relief of symptoms continued for as long as the patients were followed up, which was 17 weeks in patient 1, 12 weeks in patient 2, and 6 weeks in patient 3.
Finally, further investigation is necessary to assess the efficacy of this treatment regimen of benfotiamine and methylcobalamin in the management of peripheral neuropathy.