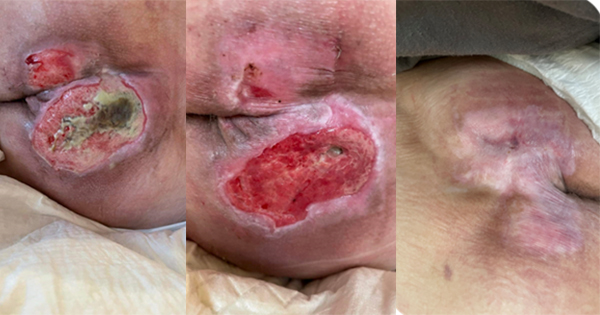
Currently, the most commonly used dressing materials for the prevention of facial medical device-related pressure ulcers/injuries are hydrocolloids and foams. Often, clinicians who choose one type over the other are unaware of the underlying differences in material behaviours, and the biomechanical considerations and implications of their selection, in particular concerning the compatibility of these dressing types with skin. Accordingly, this article aims to compare the suitability of hydrocolloids versus foams for the specific purpose of facial medical devicerelated pressure ulcer prevention, based on biomechanical considerations which are explained here in a non-technical language. In particular, the alleviation of localised and sustained tissue loads are the most fundamental requirement from any type of prophylactic dressing, and avoiding sharp stiffness gradients between the skin and the protecting dressing serves this purpose. The compressive stiffness matching ratio explained here is an intuitive and easy-to-implement biomechanical performance measure of this skin-dressing stiffness gradient. Specifically, the compressive stiffness of a dressing used for prophylaxis and the compressive stiffness of the skin region covered by the dressing are the most important and relevant properties to consider, given the common techniques of device attachment to skin which apply localised, intense compressive forces to the skin, such as for ventilation masks that are strapped to the head. Based on the above criterion, hydrocolloids exhibit poor biomechanical prophylactic efficacy in protecting healthy skin, and more so, in preventing injuries in fragile or aged skin. Foams, on the other hand, have stiffness properties that closely resemble those of human skin, and, though foam dressings by different manufacturers vary in their specific stiffness properties, some low-stiffness foams provide a nearideal stiffness matching with skin. Wound care professionals should adopt objective, standardised and quantitative research-based approaches in their clinical decisionmaking processes, to grade and then select the optimal dressings for prophylaxis of injuries caused by medical devices. This article discusses an example basic bioengineering measure, i.e., the stiffness matching ratio, which should be demanded by clinicians and disclosed by manufacturers for best-practice.