Pressure injuries (PIs) are an unfortunate persistent problem in healthcare. PIs represent one of the most challenging clinical problems that negatively impacts patients’ lives emotionally, psychologically, physically and socially, and overall decreases their quality of life (Shiferaw et al 2020; Roussou et al, 2023) and their prognostic, while also increasing the workload and financial burden on healthcare globally (Siotos et al, 2022). In fact, PIs often result in long and repeated wound care procedures, possible recurrent hospitalisations, multiple surgeries, potentially devastating complications, morbidity and early mortality (Shiferaw et al 2020).
These afflictions have been persistent for millennia. Probably the first evidence of a PI was found in Egyptian mummies, some of which are more than 5,000 years old (Agrawal and Chauhan, 2012). Literature about PIs also dates back thousands of years, where Hippocrates (460–370 BCE) associated PIs with paraplegia and bladder and bowel dysfunction, and Avicenna (980–1037 CE) provided suggestions regarding local treatment for healing of PIs (Agrawal and Chauhan, 2012). Florence Nightingale stated that “If a patient is cold, if a patient is feverish, if a patient is faint, if he is sick after taking food, if he has a bed-sore [sic], it is generally the fault not of the disease, but of the nursing” and suggested in a letter to a family with a bed-bound child that “a few very small pillows … placed here and there and moved about whenever there seems to be pressure are really preferable” (Nightingale, 2010).
Despite long-standing knowledge of the condition, prevalence and incidences of PIs is still high, notably due to an ageing population and a rise in the number of individuals living with chronic conditions, disabilities and comorbidities (Rapetti et al, 2023). A global systematic review and meta-analyses with 39 eligible studies, involving a total sample of 2,579,049 patients, estimated pooled prevalence at 12.8% and pooled hospital-acquired PI rate of 8.4% (Li et al, 2020). PIs are also associated with a substantial financial burden and a strain on resources: in the UK, the annual cost of PIs to the NHS was estimated to be between £507 and £530.7 million (Guest et al, 2018), while in the US, the annual societal cost of managing these wounds was estimated in 2016 at $26.8 billion (Gefen, 2018).
Pathophysiology
The National Pressure Injury Advisory Panel (NPIAP) definition for PIs is “localised damage to the skin and underlying soft tissue usually over a bony prominence or related to a medical or other device. The injury can present as intact skin or an open ulcer and may be painful. The injury occurs as a result of intense and/or prolonged pressure or pressure in combination with shear. The tolerance of soft tissue for pressure and shear may also be affected by microclimate, nutrition, perfusion, comorbidities and condition of the soft tissue” (Lange, 2020).
PIs are described as Stage 1: intact skin with non-blanchable erythema; Stage 2: partial-thickness skin loss involving the epidermis and dermis; Stage 3: full-thickness loss of skin that extends to the subcutaneous tissue but does not cross the fascia beneath it. Slough or eschar may be visible, and the lesion may be foul-smelling; Stage 4: full-thickness skin loss that extends through the fascia with considerable tissue loss — there may be muscle, bone, tendon, or joint involvement; Unstageable: depth is unknown because slough or eschar obscures the extent of tissue damage (Zaidi and Sharma, 2020).
The skin and underlying tissue damage is caused when an area of skin is placed under constant pressure and/or shear for a certain period, causing tissue ischaemia, cessation of nutrition and oxygen supply to the tissues with eventual tissue necrosis. Tissue distortion results from soft tissues that are compressed and/or sheared (Bhattacharya and Mishra, 2015). Blood vessels within the distorted tissue are compressed, angulated, or stretched out of their usual shape and blood is unable to pass through them (Bhattacharya and Mishra, 2015). Only minimal external pressure is needed to occlude capillaries. The average mean capillary pressure equals to about 17mm Hg, and any external pressures exceeding this will cause capillary obstruction. The tissues that are dependent on these capillaries are deprived of their blood supply and eventually the ischaemic tissues will die (Dziedzic, 2013).
Management of PI wounds
Management of PI wounds is multifactorial and depends on the site, stage and associated complications of the ulcer (Boyko et al, 2020). This incorporates patient holistic management, minimising/offloading pressure and wound management according to the local environment of the wound and the objective of the treatment (balancing moisture, removal of necrotic tissue, controlling bacteraemia). Dressings should be chosen depending on the wound being treated (Boyko et al, 2020).
A widely used method of treating wounds is based on the different wound bed preparation paradigms, including DIME (Debridement/devitalised tissue, Infection or inflammation, Moisture balance, wound Edge preparation and wound depth), which has evolved to include a holistic patient-centred approach to wound care (Snyder et al, 2016).
Debridement is the process of removal of nonviable tissue from a wound. The occurrence of nonviable tissue in the wound is seen as a factor that delays healing of PIs by preventing the generation of healthy granulation tissue, while also providing a good environment to harbour more microorganisms, thereby increasing the risk of further sepsis (Stansby et al, 2014). The choice of debridement method depends upon the nature of the wound, the skill set of the practitioner, access to equipment and dressings and the condition of the individual (Stansby et al, 2014). Slough, comprising fibrin, leucocytes, dead and living cells, microorganisms and proteinaceous material, is a common feature of chronic wounds (Angel, 2019). The appropriate and safe removal of slough is a necessity for PIs to heal, as it not only interferes with staging and assessment, but it also contributes to delayed or stalled wound healing (Angel, 2019).
Moisture balance has been shown to be beneficial to wound healing since the cornerstone studies by George D Winter (1962) and Hinmann and Maibach (1963). Balanced moist wound environments enhance wound healing by promoting angiogenesis and new tissue growth, fibroblast proliferation and collagen synthesis, therefore reducing pain and reducing scarring (Guo and DiPietro, 2010).
Possible infectious complications of PIs are abscess, osteomyelitis and high frequency of bacteraemia, which may lead to significant mortality. Local infection of PIs is usually polymicrobial and the chances of colonisation by new microorganisms are high (Espejo et al, 2018). PIs can be colonised with Gram-negative bacilli and can be reservoirs for multidrug-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA). Colonisation may evolve into local infections, also becoming a source of bacteraemia, which may also lead to the development of biofilms (Suleman and Percival, 2014; Braga et al, 2017). Biofilm microorganisms are tolerant to the action of a wide range of antimicrobials due to the protection provided by the extracellular polymeric substances (Suleman and Percival, 2014). It is therefore obvious that the common presence of a biofilm in PIs proves to be more challenging and necessitates a different approach to the treatment and management of these wounds (Suleman and Percival, 2014).
Finding dressings that can provide the clinician with all the beneficial factors to target these challenges can be quite difficult. Healthcare professionals recognise the need for reliable evidence to guide practice and that traditional techniques, which experience suggests are likely to be helpful, may not provide the best option (Chapman, 2017). Evidence-based decisions do matter, guiding clinicians in knowing what is most likely to be effective, which dressings to apply, including how and when to use them and for which wound. Moreover, it is important to understand what best available evidence says in order to advocate for its use (Chapman, 2017).
Evidence-based approach
Myriad dressings and devices are available for clinicians to improve healing rates and patient outcomes, but, there is a need to critically appraise evidence to make appropriate and effective evidence-based changes to practice (World Union of Wound Healing Societiers, 2020). As established in the ground roots of evidence literature from Sachet et al (1996), it is evident that, without best evidence, standards of patient care are not maintained.
Unfortunately, high-quality evidence pertaining to pressure wound management is limited (Welsh, 2018). However, some modern advanced dressings have been shown, though robust evidence, to be beneficial in the removal of necrotic tissue and fibrin, can play a role in autolysis and debridement, avoid re-injury of new granulation tissue, and promote cell proliferation, differentiation and epithelial cell migration (Shi et al, 2020).
Dressing under evaluation — UrgoClean Ag
UrgoClean Ag is an advanced wound care dressing made of cohesive poly-absorbent fibres impregnated with a silver lipido-colloid matrix (Technology Lipido-Colloid-Ag healing matrix [TLC-Ag]). The different technologies included in this dressing have all been rigorously tested in in vitro and in vivo studies, and clinical evaluations to provide an evidence-based solution for wound care clinicians.
Technology Lipido-Colloid (TLC) was developed by Laboratoires URGO (Chenôve, France). It comprises a matrix containing hydrocolloid and lipophilic substances. The TLC matrix has been shown to promote fibroblast proliferation, which in turn also contributes to the formation of new tissue (Bernard et al, 2005; McGrath et al, 2014; White 2015). The hydrocolloid polymers are hydrated when in contact with the wound exudates and bind with the lipidocolloid interface, thus reducing adhesion to the wound surface (Meaume et al, 2002), allowing atraumatic removal (Meaume et al, 2004).
With the incorporation of silver sulphate (3.5%) to the TLC healing matrix, TLC-Ag provides antimicrobial properties (White et al, 2015). The antimicrobial efficacy of the dressing was assessed in an in vitro study that showed, from the first hours and throughout the duration of the seven-day study, reduction in the number of colony-forming units for all the bacterial strains studied, including MRSA and Vancomycin-resistant Enterococcus (VRE) (White et al, 2015). The efficacy of the TLC-Ag was reported as having the ability to destroy Staphylococcus aureus and Pseudomonas aeruginosa biofilms and to have antimicrobial effects on numerous organisms (Quatravaux et al, 2008; White et al, 2011) and to have significant anti-inflammatory effects on chronic skin inflammation in vivo (Bisson et al, 2013). The efficacy of the TLC-Ag has also been demonstrated in a multicentre, phase III, controlled, randomised trial, compared to dressings without silver, in the management of the wound bioburden and promoting wound healing (Lazareth et al, 2012).
More recently, the TLC-Ag was also studied in a prospective, multicentre study on 728 patients in 39 centres for a mean duration of 26±19 days, with wounds at risk of or with local infection, under real-life conditions during the COVID-19 pandemic (Lützkendorf et al 2022). It was determined that, throughout the study period, all the parameters of wound infection progressively decreased, and by the final visit there was a reduction by 78.9% of the prevalence of local wound infections and by 72.0% of the clinical signs of wound infection. Simultaneously, in terms of the healing process, 92.1% of the wounds healed or improved, 3.2% remained unchanged and only 1.7% worsened (data missing for 3.0%), with an improvement of the peri-wound skin reported in 65.7% of the patients. The efficacy of TLC-Ag was also recently portrayed in clinical case series from Asia, with very encouraging results (Uppal et al, 2020; Yingtao et al, 2021; Qiuju et al, 2020).
The poly-absorbent fibres are beneficial in the absorption of wound exudate as well as the trapping of sloughy residue (Meaume et al, 2012; Sigal et al, 2019). The desloughing properties of the poly-absorbent fibres have been confirmed in a randomised controlled trial involving 159 patients to be superior to Hydrofiber (Meaume et al, 2014). An earlier non-comparative evaluation stipulated that UrgoClean® (poly-absorbent fibres without silver) markedly reduced slough in a cohort of 44 patients with chronic venous leg ulcers and Stage 3 and 4 PIs (Meaume et al, 2012). Results following 6 weeks of treatment with UrgoClean® showed a decrease in sloughy tissue (75% and 89% in the venous leg ulcers and PIs, respectively), total healing in six wounds, marked reduction of surface area (mean 23.7% ± 53.4% [leg ulcers] and 29.2% ± 72.5% [PIs]), improvement of the peri-wound skin, and high rankings in acceptability, conformability and pain-free removal.
Clinical evidence
UrgoClean Ag is also supported by high-quality clinical evidence in the management of wounds at risk or with clinical signs of local infection (Dissemond et al, 2020). UrgoClean Ag has essential abilities to isolate and immobilise microbes both within the free-floating planktonic state and when they have been detached from the biofilm (Percival, 2020). The poly-absorbent fibres help in continuously cleaning the wound by the affinity of the fibres to the wound debris, and the silver component provides the desired antimicrobial effect (Percival, 2020).
The combined cleaning and antimicrobial action contribute to the third mode of action of the dressing, that is, the achievement of effectively disrupting biofilm (Percival, 2020). In vitro studies showed the deeper penetration of the ionic silver into the biofilm acting as bactericidal to the microorganisms found within it after 24 hours of exposure (Percival, 2018, Figure 1).
Other in vitro investigations established the synergic action of the TLC-Ag matrix and the poly-absorbent fibres against MRSA and Pseudomonas aeruginosa biofilms (Desroche et al, 2016; Desroche and Dropet, 2017). The application of UrgoClean Ag resulted in a significant decrease of the biofilm population by a log reduction of 4.6, after 24 hours of exposure, which was maintained for seven days, with reduction values up to 4.0 log (reduction of biofilm superior to 99.99%) (Desroche et al, 2016).
The antibiofilm activity of UrgoClean Ag was also compared to that of a carboxymethylcellulose (CMC) dressing, which combines ionic silver, a metal chelating agent, and a surfactant (Hydrofiber Ag + Extra) (Desroche and Dropet, 2017). In this in vitro model, after 24 hours of exposure, UrgoClean Ag demonstrated higher anti-biofilm activities than the CMC dressing. In these conditions, the anti-biofilm efficacy of the polyabsorbent silver dressing was shown to be 50 times stronger on MRSA biofilm and 100 times stronger on P. aeruginosa biofilm than the CMC dressing.
The first clinical study assessing UrgoClean Ag dressings was a prospective multicentre non-controlled clinical trial conducted in France, including 37 patients with chronic wounds from 17 active investigating centres, treated for a maximum period of four weeks with the evaluated dressing and followed by the physician on a weekly basis (Dalac et al, 2016). The wound surface area, mostly covered by sloughy tissue at baseline, was reduced by 32.5% at the final visit. Effective debridement was achieved, with 62.5% relative reduction of sloughy tissue and 58.8% of debrided wounds at week 4. Improvement of the periwound skin status was also documented with 28.6% of wounds at week 4 versus having a healthy periwound versus 2.7% at baseline. Moreover, UrgoClean Ag presented a good safety profile with high level of acceptability by both patients and clinicians.
A large, real-life, multicentre, observational study of 2,270 patients with acute and chronic wounds of various aetiologies was conducted in 81 centres in Germany (Dissemond et al, 2020). The patients, including 77 children and 118 PIs (one in a minor), presented with exuding wounds at risk of infection or with clinical signs of local infection. These wounds were managed with UrgoClean Ag for a mean duration of 22±13 days. All clinical signs of local infection and diagnosed wound infections were substantially reduced at two weeks after treatment initiation, and all wound infection parameters continued to reduce until the last visit. The number of diagnosed wound infections reduced from 318 (14.0%) at baseline, to 70 (3.1%) after 2 weeks of treatment, at the interim visit, and to 32 (1.4%) by the final visit (week 3). Similar results were noted, notwithstanding exudate level and proportion of sloughy and granulation tissues in the wound bed at baseline. The authors stated that the evaluated dressing promoted the wound healing process. Regarding the 118 patients with a PI at risk and/or with clinical signs of local infection, after 4 weeks of treatment, 33.1% healed and 62.7% improved (from 10.6 cm² wound area at baseline to 3.4cm², on average), with a very good or good acceptance by 96.6% of the patients (data on file).
Clinical cases of patients from Asia managed by UrgoClean Ag are also available. Hieu DV et al (2021) documented four diabetic foot ulcers, one venous leg ulcer, two traumatic wounds and one burn case, managed successfully with the dressing. The authors concluded that “UrgoClean Ag can be considered as one of the important introductions to the clinicians’ toolbox” and that similar results to European cases can be replicated in Asia. Seshabhattaru et al (2021) also reported positive results in 20 diabetic foot ulcers, three venous leg ulcers, one post-operative case of craniopharyngioma (2-year-old patient) and one case of Fournier’s gangrene. The authors noted the dressing’s fast and effective antimicrobial action and complete and continuous cleaning action to remove slough, reduce exudate and bacterial residues, and ability to destroy and remove biofilms. UrgoClean Ag was considered as a dressing that is easy to use, more so that some patients had been able to change their own dressings at home. Patients have found the dressing comfortable during wear time and atraumatic and pain-free at removal. Nguyet TTA et al (2022) also documented the use of UrgoClean Ag in nine burn cases, including two 3-year-old children and one 6-year-old, again with very positive results.
The cases
In view of the robust and consistent body of evidence regarding UrgoClean Ag and its benefits, the authors determined to evaluate UrgoClean Ag in cases of PIs that they encounter frequently in their practices. The dressing was part of their evidence-based holistic standard of care for patients with PIs.
Case 1
Dr Abhishek Tiwari, New Delhi, India
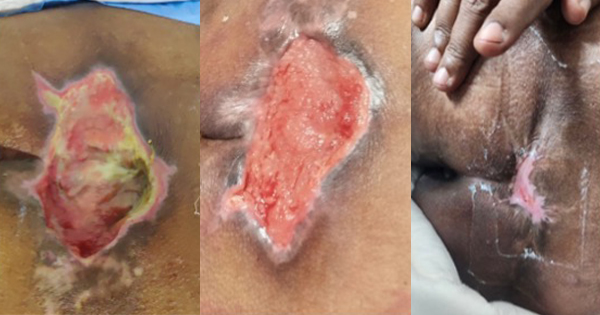
A 93-year-old female, bedridden for around 7 years, with history of hypertension and dementia, had a Stage 4 pressure injury (PI) over the sacral region that had been regressing for 3 months [Figure 2A], while previously managed with traditional wound care. The wound measured 10 x 7 x 5cm, sloughy and heavily exuding, macerated periwound skin, with a pain score of 5 (out of 10).
A decision was taken to change the dressing regime to cleansing with normal saline and application of UrgoClean Ag® as primary dressing with a secondary TLC sacral foam border dressing. Dressing was changed every 3–5 days, depending on strikethrough. A pressure injury standard of care was also implemented, including offloading, hourly turnings, nutritional support and placing the patient on an active air mattress.
By week 4, progress was noted in the wound bed with healthy granulating tissue covering most of the wound bed, healthy peri-wound, reduction in exudate levels, reduction in pain score to 4 and reduction of wound size (8 x 4 x 3cm) [Figure 2B]. By week 9, further improvement was documented, with the wound now measuring 5 x 3 x 2cm, the wound bed mostly covered with healthy granulating tissue, surrounding by healthy periwound skin, and further reduction of exudate level and pain intensity (score of 2) [Figure 2C]. Within 2 more weeks, the wound was almost completely healed (2 x 2cm), fully granulated, healthy peri-wound skin and no exudate or pain. Complete healing was achieved by week 14 [Figure 2E].
After the application of the new protocol, there was a rapid and substantial reduction in exudate levels, improvement of granulation tissue and complete elimination of sloughy tissue, with reduced pain scores and final wound closure.
Blood silver levels were monitored during the treatment to monitor a potential toxicity and results were within normal range.
Cases 2 and 3
Dr Riju R Menon, Professor, and Dr Anoop Vasudevan Pillai, Cochin, India
A 78-year-old female, who had been bedridden for 3 years, known case of Type II diabetes mellitus, hypertension, Alzheimer’s disease, hypothyroidism and dyslipidaemia, had been referred with two 2-month-old Stage 4 PIs, one over the sacral region and one on the left trochanteric region [both Figure 3A] that worsened after being managed with traditional wound care. Both wounds were cleansed with normal saline and UrgoClean Ag was used as primary dressing with a secondary absorbent dressing. A pressure injury standard of care was also implemented including offloading, regular turnings, nutritional support and placing the patient on an active air mattress.
The sacral PI measured 20 x 15 x 5cm, presented with a sloughy wound bed, heavy exudate and elevated pain (score of 7 out of 10). Dressing was changed on average every 5 days. By week 2 and week 4, the wound had shown good progress [Figure 3B and Figure 3C, respectively], with the wound presenting with mostly healthy granulating tissue, reduction in exudate levels and pain intensity (score of five). By week 5 [Figure 3D], the wound size had reduced to 15 x 11cm, wound bed covered with healthy granulating tissue, and further reduction of exudate levels. By week 7, the wound had almost healed with good epithelisation [Figure 3E].
On referral, the Trochanteric PI measured 10 x 8 x 1.5cm, with heavy exudate and a pain score of 7 (out of 10). The same management was applied for this wound, and, within 2 weeks [Figure 4B], the wound was progressing with a decrease in size (9 x 7 x 1cm) and an increase in granulation tissue. By weeks 4 and 5, the wound continued to progress [Figure 4C, 4D, respectively]. By the 5th week, the wound size had reduced to 4 x 3 x 1cm and further reduction in exudate levels and pain score (five). The wound was healed by week 7 [Figure 4E].
The dressing protocol aided in rapidly desloughing the wounds, and reducing exudate levels, pain score wound surface area and wound volume, respectively.
Blood silver levels were monitored during the treatment to monitor a potential toxicity and results were within normal range.
Case 4
Nguyen Viet Dung, RN, Ha Noi City, Vietnam
A 75-year-old female was referred for management of a 20-day-old, 5 x 4cm sacral unstageable pressure injury with high levels of exudate, previously untreated [Figure 5A]. The patient had a past medical history of cerebrovascular accident (3 months prior), pneumonia and tracheostomy, and was bedridden since her cerebrovascular accident.
The wound was cleansed with normal saline and sharp debridement was done, and UrgoClean Ag was used as a primary dressing with a secondary absorptive dressing. Systematic antibiotics were started 10 days before admission and continued throughout ulcer treatment. Dressing was changed daily. Position turning, nursing the patient on an alternating air mattress, and nutrition and parenteral albumin supplements were initiated as part of holistic management of the PI.
By the fourth day, the wound bed was looking healthier and exudate levels had decreased [Figure 5B]. By day 12 [Figure 5C], the exudate levels had further decreased to low levels and therefore it was decided to change the dressing every third day. By day 27, the wound bed was almost completely covered with healthy granulation tissue, exudate levels continued to be low, and thereafter the dressing was changed every four days [Figure 5D]. At this point, the patient was transferred to a step-down facility.
The new dressing protocol helped in reducing slough and exudate and rapidly promote healthy granulation tissue.
Case 5
Nguyen Viet Dung, RN
A 74-year-old male with a past medical history of hypertension and Chronic Obstructive Pulmonary Disease, was referred for a seven-day-old Stage 3 untreated pressure injury in the sacral region, measuring 12 x 8cm, almost completely covered with slough and producing high levels of exudate [Figure 6A].
The wound was cleansed with normal saline and sharp debridement was done [Figure 6B], and UrgoClean Ag was used as the primary dressing with a secondary absorptive dressing. Systematic antibiotics were also started at this point for a duration of 1 week. Dressing was changed daily. Position turning, nursing the patient on an alternating air mattress and nutritional supplements were initiated as part of holistic management of the pressure injury.
By day 14, there was improvement of the wound bed with a marked reduction in exudate from high to moderate levels [Figure 6C], and by day 21, the slough was almost completely eliminated with healthy granulating tissue covering the wound, and exudate levels being now low [Figure 6D]. The patient was thereafter management in the community.
In this instance, with a very large wound, the new dressing protocol again helped in reducing slough and exudate and promote healthy granulation tissue.
Case 6
Nguyen Viet Dung, RN
A 98-year-old female with a medical history of schizophrenia, diabetes mellitus T2 and cerebrovascular accident (eight months earlier and bedridden ever since) was referred for management of a 3-week-old, highly exudative Stage 4 PI in the sacral region, which had previously been managed with gauze soaked in povidone iodine [Figure 7A].
Surgical debridement was done, the wound was cleansed with normal saline and UrgoClean Ag was used as primary dressing with a secondary absorptive dressing. Systematic antibiotics were also started at this point and were stopped after 10 days. Dressing was changed daily. Position turning, nursing the patient on an alternating air mattress, and nutrition and parenteral albumin supplements were initiated as part of holistic management of the PI.
By day eight [Figure 7B], the exudate levels were greatly reduced to low levels, with the wound bed mostly covered with healthy granulating tissue. Further improvement and lower exudate levels and healthier wound bed were reported by day 12 [Figure 7C]. Unfortunately, the patient later died due to her very precarious state of health.
In this wound in an older patient, the new dressing protocol again provided good results in reducing slough and exudate and promote healthy granulation tissue.
Case 7
Dang Thi Cam Van, RN, Orthopaedic Department, Khanh Hoa General Hospital, Nha Trang City, Vietnam
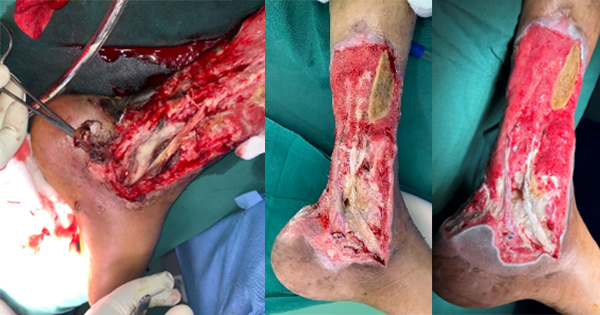
A 79-year-old male patient, bedridden since 19 months, with a known medical history of hypertension, was refereed for the management of an unstageable PI in the sacral region, measuring 20 x 20cm, and producing low exudate. The pressure injury occurred about three months before hospitalisation and was previously managed by the carer at home.
The wound was cleansed with normal saline and UrgoClean Ag was used as primary dressing with a secondary absorptive dressing. Systematic antibiotics were also started at this point and continued for 2 weeks. Dressing was changed alternate days. Position turning, nursing the patient on an alternating air mattress and nutritional supplements were initiated as part of holistic management of the pressure injury.
By day 25, the wound bed was looking healthier, with slough almost entirely eliminated, and wound producing lower levels of exudate [Figure 8B]. By day 61, the wound area was substantially reduced (10 x 5cm) and covered with healthy granulation and epithelialisation tissue [Figure 8C]. At this point the patient was discharged and the wound was managed in the community with a non-adherent TLC-Ag dressing.
The management of this large wound produced impressive results with the wound area greatly reduced and epithelialising.
Discussion
PIs are chronic wounds that are difficult to treat and that tend to recur after healing (Mervis and Philips, 2019). Overcoming the factors that contribute to delayed and prolonged healing is a key component of a comprehensive standard of care in wound care and presents the primary challenge to the treatment of chronic wounds (Frykberg and Banks, 2015). Among other challenges, clinicians need to address nonviable tissue, infection and biofilm when managing these wounds and understanding and tackling these challenges will result in better clinical outcomes, leading to an improved patient quality of life and reduced healthcare costs (Frykberg and Banks, 2015).
The evidence behind UrgoClean Ag is extensive, including the results from 119 patients with PIs reported in clinical trials and real-life studies (Dalac, 2016; and Dissemond, 2020). Although this is a considerable number, the authors intended to independently evaluate UrgoClean Ag dressings as part of their already existing standard of care in the management of their challenging PI cases, to assess its efficacy in these serious cases. Results showed effective and rapid improvement in these cases too. In all the cases, the wound surface area was reduced and nonviable tissue removed, in line with the dressing strategy expectations. These results are also in line with the aforementioned clinical studies conducted in Europe and cases published from Asia, supporting that UrgoClean Ag is also an effective dressing in the management of chronic wounds.
Conclusion
With so many different dressings available, it is imperative that clinicians choose wisely in accordance with available good quality evidence. UrgoClean Ag is supported in vitro but also by clinical trials and real-life studies. The technologies incorporated in this dressing have shown to be of great benefit for clinicians to provide continuous cleaning action, as well as an antimicrobial and anti-biofilm solution.
By publishing these challenging cases, the authors strived to share their positive experiences achieved with the dressing in PIs and propose to other clinicians to evaluate UrgoClean Ag in their own settings. The inclusion of the dressing with an evidence-based standard of care may provide better management of these wounds.