A 59-year-old female with diabetes presented with a hypergranulated ulcer on her left index finger from a dog bite that occurred approximately 6 months prior to her first visit to the wound clinic. She was a nurse and was caring for it by herself, but when it got larger, she made an appointment at the wound care clinic.
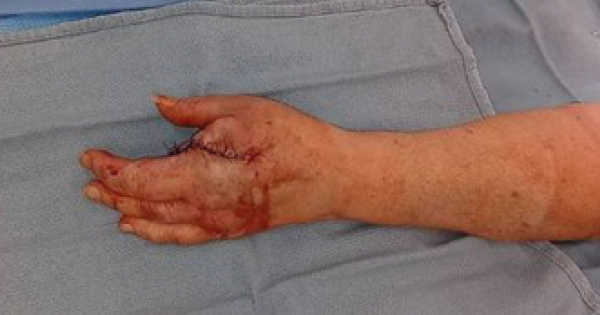
Her vital signs were all within normal range. She had a large hypergranulated ulcer on the tip of the left index finger and the tuft of the distal phalanx was palpable in the ulcer [Figure 1].
Two punch biopsies were performed; one for tissue culture and one for surgical pathology. The culture results were reported first and were positive for moderate MRSA. She was prescribed doxycycline. The surgical pathology showed invasive amelanotic melanoma, which is a form of melanoma in which the malignant cells have little to no pigment. She was referred to hand surgery for evaluation and treatment. She had a PET scan, which showed no evidence of regional lymph node or distant metastasis. She subsequently had a ray resection of the left first metacarpal [Figure 2] followed by a sentinel node biopsy in the left axilla and it was negative for malignancy.
Marjolin’s ulcer is classically referred to as a squamous cell carcinoma arising in burn scars, but it is also used to describe aggressive malignant degeneration in any chronic wound (Tobin and Sanger, 2015). It was described for the first time in 1828 by Jean-Nicolas Marjolin (Visuthkosol et al, 1986; Aron and Tajuri, 1989; Dupree et al, 1998; Copcu and Culhici, 2002; Pandey et al, 2009). It occurs mostly in the fifth decade of life with a higher preponderance in men, and usually years or decades after the initial injury (Oruç et al, 2019). It can occur in all anatomical locations, but mostly in the lower extremities (>40%), followed by upper extremities, head, neck and trunk (Tania, 1998). They are rarely encountered in the digits (Holgado et al, 2000). In this case series by Holgado et al, there were no instances of melanoma (Holgado et al, 2000).
Marjolin’s ulcers are squamous cell carcinoma (SCC) in 95% of cases, basal cell carcinoma in 2–3% of cases, and melanomas and sarcomas develop very rarely, with melanoma occurrence listed at approximately 2.4% (Spring et al, 2001; Serras et al, 2019; Saeed et al, 2019). Although melanomas are defined as the third most frequent diagnoses, there are only two melanoma cases that developed from burn scars in the literature (Flemming et al, 1990; Türegün et al, 1997; Gargan et al, 1988; Soto-Dávalos et al, 2007).
There is a report of acute Marjolin’s ulcer in the literature, which presented as squamous cell carcinoma from a dog bite(also in the left index finger) (Henderson et al, 2021). There was another report of amelanotic nodular Marjolin’s ulcer on the left foot following trauma, but while the time lapse between the trauma and the discovery of the Marjolin’s ulcer is not documented, it references the progressive growth of the lesion over a period of 2 years (De Ikuverua et al, 2023).
Diagnosis
The diagnosis of Marjolin’s Ulcer is based on assessment of patient’s history, detailed examination of the ulcer and histology of the lesion (Saaiq and Ashraf, 2014). Clinical signs suggestive of Marjolin’s Ulcer include everted or rolled margins, exophytic granulation tissue formation, increasing size, bleeding and regional lymphadenopathy (enlarged lymph glands proximal to the lesion – in this case, axillary) (Copcu and Culhici, 2002; Pekarek et al, 2011). The current standard diagnostic test for Marjolin’s Ulcer is histologic analysis via biopsy (Tobin and Sanger, 2015; Meaume et al, 2013). Computerized tomography (CT) and/or MRI may provide the degree of soft tissue involvement and clinical examination of regional lymph nodes is necessary (Khan et al, 2020).
Treatment
The mainstay of treatment for Marjolin’s Ulcers is wide local excision. Oncologic clearance entails excision of the lesion with 2–4 cm horizontal clearance margins. There is no established consensus on the adjuvant or neoadjuvant management (radiation or chemotherapy) (Saaiq and Ashraf, 2014).
Prognosis
The subtle presentation of Marjolin’s Ulcers often causes a delay in diagnosis and treatment. The overall mortality rate for Marjolin’s Ulcers is 21% (Saaiq and Ashraf, 2014). Overall, Marjolin’s ulcers have a reported metastatic rate between 27.5% and 40% (Iqbal et al, 2015). Poorer prognostic clinical features in Marjolin’s ulcer include regional nodal spread, local extension of the lesion, lower limb lesions (as these have a greater propensity for nodal involvement), infiltrative variety, primary lesions of ≥2 cm, latency period of ≥5 years, recurrence, and presence of distant metastasis (Saaiq and Ashraf, 2014). This patient had no evidence of metastatic disease; therefore, the patient needed no further treatment.
Discussion
This article suggests the importance of a high index of suspicion for any wounds where the initial history or examination have an unusual presentation and during a course of care, the wound does not appear to be healing in an expected manner.
Conclusion
The most frequent wound or scar type causing malignancies is burns (Ozek et al, 2001). Most Marjolin’s’ Ulcers have a long latency period between the initial injury and the appearance of the malignancy. Acute Marjolin’s ulcers are rare, and those with a histologic diagnosis of melanoma are even rarer.
The importance of an adequate history, awareness of any atypical appearing ulcer, and appropriate diagnostic testing (e.g., biopsy at initial presentation) will help alleviate delays in diagnosis of Marjolin’s ulcers allowing for prompt definitive treatment and improved prognosis.