Providencia rettgeri is a Gram-negative bacillus that belongs to the Enterobacteriaceae family. It is commonly considered as a commensal microorganism which colonises the human gastrointestinal tract and is ubiquitous in soils, water and animal reservoirs (Washington et al, 2015; Huff and Blome-Eberwein, 2022).
There are currently five species in the genus Providencia: P rettgeri, P stuartii, P alcalifaciens, P rustigianii and P heimbachae. P stuartii is the most prevalent and has been associated with post-burn wound infections (Huff and Blome-Eberwein, 2022).
P rettgeri, as an opportunistic pathogen, is most usually seen in urinary tract infections (UTI; Huff and Blome-Eberwein, 2022). It was first isolated in 1904 by Rettger in a fowl cholera-like epidemic in chickens, and the first documented case of P rettgeri in humans was published in 1951 by Goldfarb and De Bakey, who reported an empyema-associated infection (Goldfarb and De Bakey, 1951; O’Hara et al, 2000; Washington et al, 2015). It is rarely associated with human disease, although has gained recognition in the past few years as an emerging pathogen in urinary tract and catheter-related, bloodstream, respiratory, traveller’s diarrhoea and seldom, skin and soft tissue infections (Yoh et al, 2005; Washington et al, 2015; Huff and Blome-Eberwein, 2022). Infrequently, it has also been associated with ocular infections and “Purple Bag Syndrome”, in which urine becomes purple as consequence of enzymatic bacterial degradation (Al-Jubouri and Vardhan, 2001; Koreishi et al, 2006).
P rettgeri produces biofilm, being usually resistant to commonly used antibiotics like aminoglycosides, such as gentamicin and tobramycin, although it has some susceptibility to amikacin (O’Hara et al, 2000; Sapkota et al, 2021). There are also some studies that refer natural innate resistance to β-lactams due to the expression of β-lactamases, including third-generation cephalosporins (Huff and Blome-Eberwein, 2022).
There are increasing reports of multidrug resistance, including carbapenemases and extended spectrum. beta-lactamase-producing strains in epidemiological studies, although antibiotic-resistant strains were described as early as 1971 (O’Hara et al, 2000; Rosenberger et al, 2011; Sheng et al, 2013; Sapkota et al, 2021).
Skin and soft tissue infections with P rettgeri are rare and there are few studies or case reports described in the literature.
In this article, we present a clinical case of a leg ulcer of several months’ duration that presented in the Emergency Department.
Case report
A 43-year-old woman from Brazil presented in the Emergency Department (ED) at a hospital in Lisbon, Portugal, complaining of a wound in the right leg of 3 months’ duration.
She reported no recent travel aboard, animal bites or other trauma. The only medical history were varicose veins in her lower limbs.
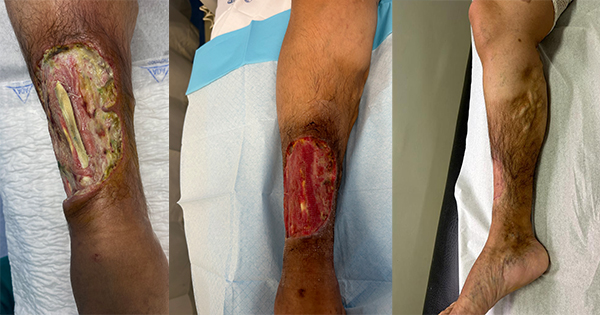
Upon physical examination, an ulcer with 15 cm × 10 cm of the anterior margin of the lower third of the right leg was objectified, with exposure of tibial crest and fibula as well as the anterior tibial tendon [Figure 1]. The arterial pulses of the limb were present and she did not perform foot dorsiflexion. She did not have any apparent sensory or other motor deficits.
According to the Clinical-Etiology-Anatomy-Pathophysiology (CEAP) classification, she had grade 1 varicose veins in the left lower limb and grade 6 varicose veins in the affected leg.
On the day she presented at ED, she was admitted and surgical wound debridement was performed. Meropenem was prophylactically initiated, and daily bandage care was started. The swabs isolated P rettgeri and Pseudomonas aeruginosa.
After the microbiological results, IV antibiotics were switched to piperacillin/tazobactam. A sample was sent to histopathology, and the wound was considered as cutaneous ulcer compatible with venous ethology due to chronic stasis.
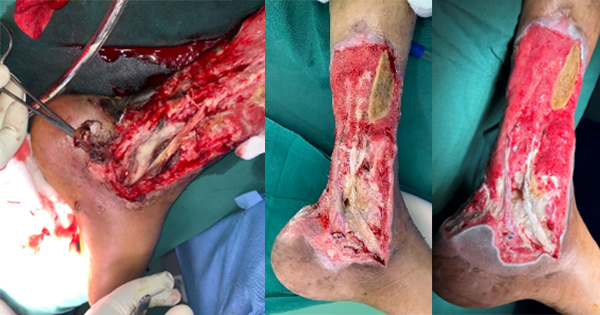
After surgical debridement and endovenous antibiotic therapy, the ulcer had favourable evolution and formed granulation tissue [Figure 2]. Doppler ultrasound revealed insufficient saphenofemoral junction and internal saphenous vein, leading to insufficient collaterals.
One month after admission, the patient was submitted to another surgical debridement and split-thickness skin grafting to achieve soft tissue coverage.
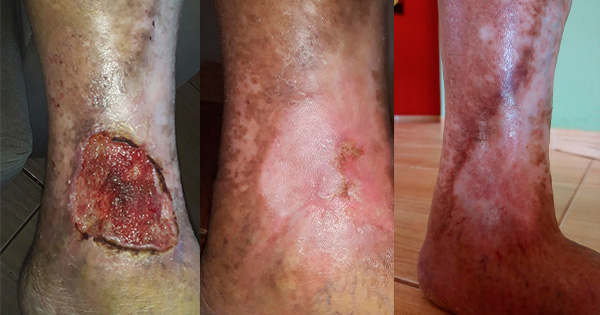
The patient was discharged 5 days after the second surgery with good healing of the wound [Figure 3].
Discussion
There are several differential diagnoses for leg ulcers and it must include venous stasis, ischemia, malignancy, infection, neuropathy, trauma or underlying systemic disease, as immunological and rheumatological aetiologies.
Given this patient’s age, the absence of comorbidities besides varicose veins and the described development of the wound, venous ethology due to chronic stasis followed by polymicrobial infection may have produced an environment where the opportunistic P rettgeri could proliferate. Venous leg ulcers are usually chronic manifestations of venous insufficiency with delayed progression over time. In this report, this was not the case.
Providencia infections in humans are rare and are usually acquired in nosocomial settings, usually implicated in UTI, bacteraemia and catheter-associated infections. Rarely, it can be seen in wound infections.
In this case report, it is unclear where the organism was acquired and how it contributed to wound pathology, since no virulence factors have been described for P rettgeri (Washington et al, 2015). It is possible that it was introduced following a skin wound exposure to contaminated water or soil. Providencia species typically manifest as polymicrobial infections, and previous colonisation with Pseudomonas, isolated in microbiological results in this case, probably enhanced infection process and ulcer development, creating a favourable environment and attenuating local immune response.
P rettgeri belongs to the Enterobacteriaceae family, which represents the first World Health Organization priority classification in the list for new antibiotic needs, stressing its importance in terms of greater understanding (WHO, 2017).
Conclusion
It is important to understand how P rettgeri affects wound pathophysiology in order to distinguish between colonisation and infection, and to establish clinical care standard and treatment goals.
The literature regarding P rettgeri skin infections is limited, and to the authors’ knowledge, only two other studies described the use of a split-thickness skin graft following an infection with P rettgeri — one in a post-burn infection and another one in a snake bite, and both reported successful outcomes (Cheong et al, 2015; Huff and Blome-Eberwein, 2022).