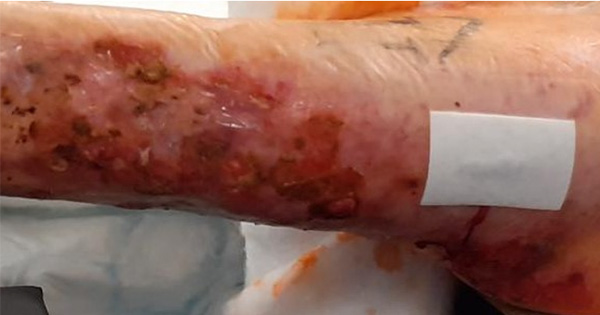
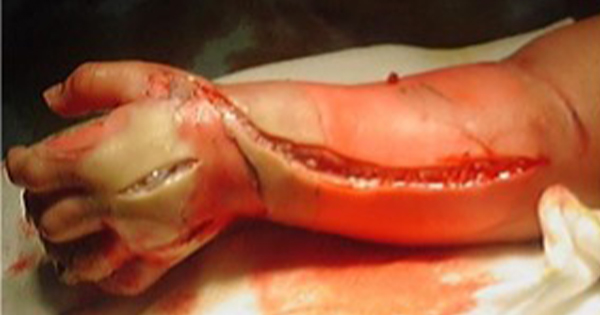
Aim: To investigate use of an advanced polyurethane foam dressing for recipient site wound healing after a split-thickness skin graft (STSG) for diabetic wound closure. Methods: Graft viability, ease of use of the dressing, exudate management and wound infection status were assessed in 18 patients who underwent a STSG for diabetic wound closure and postoperative recipient site management with a polyurethane foam dressing (BETAplast Silver, Mundipharma). Results: Graft uptake was excellent in all 18 patients (100% viability at postoperative day 30). Clinicians assessed the dressing as easy to use at various anatomical sites, with good exudate management and a low infection rate. Conclusion: BETAplast Silver dressing supports recipient site wound healing after a STSG for diabetic wound closure.