Acute Compartment Syndrome (ACS) of the limbs is a severe condition caused by the increase of the pressure within an osteofascial compartment which is transmitted to the venous system causing further transudation of fluid leading to decrease of the perfusion gradient across capillary bed and subsequent cellular anoxia and muscle ischaemia (Seileret and Olvey, 2003; Köstler et al, 2004; Gourgiotisy et al, 2007; Schmidt, 2016; Livingston et al, 2017).
In the paediatric population ACS occurs mostly in the trauma setting, as a result of bone fractures, contusions, soft tissue and muscles injuries. Other causes are fluid extravasation, external pressure, thrombosis, bleeding disorders, burns, snake and spider bites (Olson and Glasgow, 2005; Burkhartet al, 2007; Livingston et al, 2017)
ACS of the limbs due to fluid extravasation in paediatric population is an unusual and potentially devastating event, therefore, careful monitoring of the insertion site of peripheral vascular access is warranted to prevent onset. Intravenous sites should be checked at least every 2–4 hours, but the fixation dressing sometimes does not allow it.
Clinical surveillance of the patient is of paramount importance especially in newborn babies who may require immobilisation of the limb with thermoplastic splints or other devices. These devices may be responsible for the delayed recognition of extravasation and possible complications, such as compartment syndromes. This is compounded by the inability of the infant to communicate increasing pain. Early diagnosis of ACS may also be difficult in patients undergoing surgical procedures under general anaesthesia in which careful monitoring of peripheral vascular access is required, especially when pressurised fluid infusion is used (Tobias et al, 1991; Willsey and Peterfreund, 1997; Edwards et al, 2003).
Materials and methods
Case 1
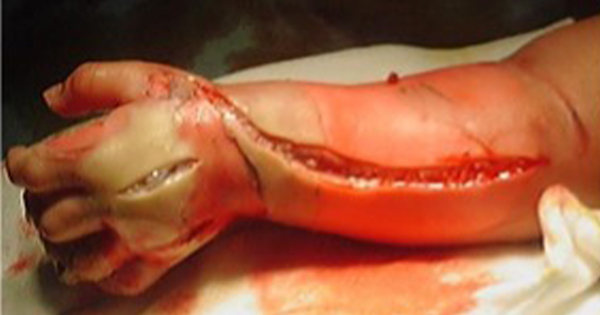
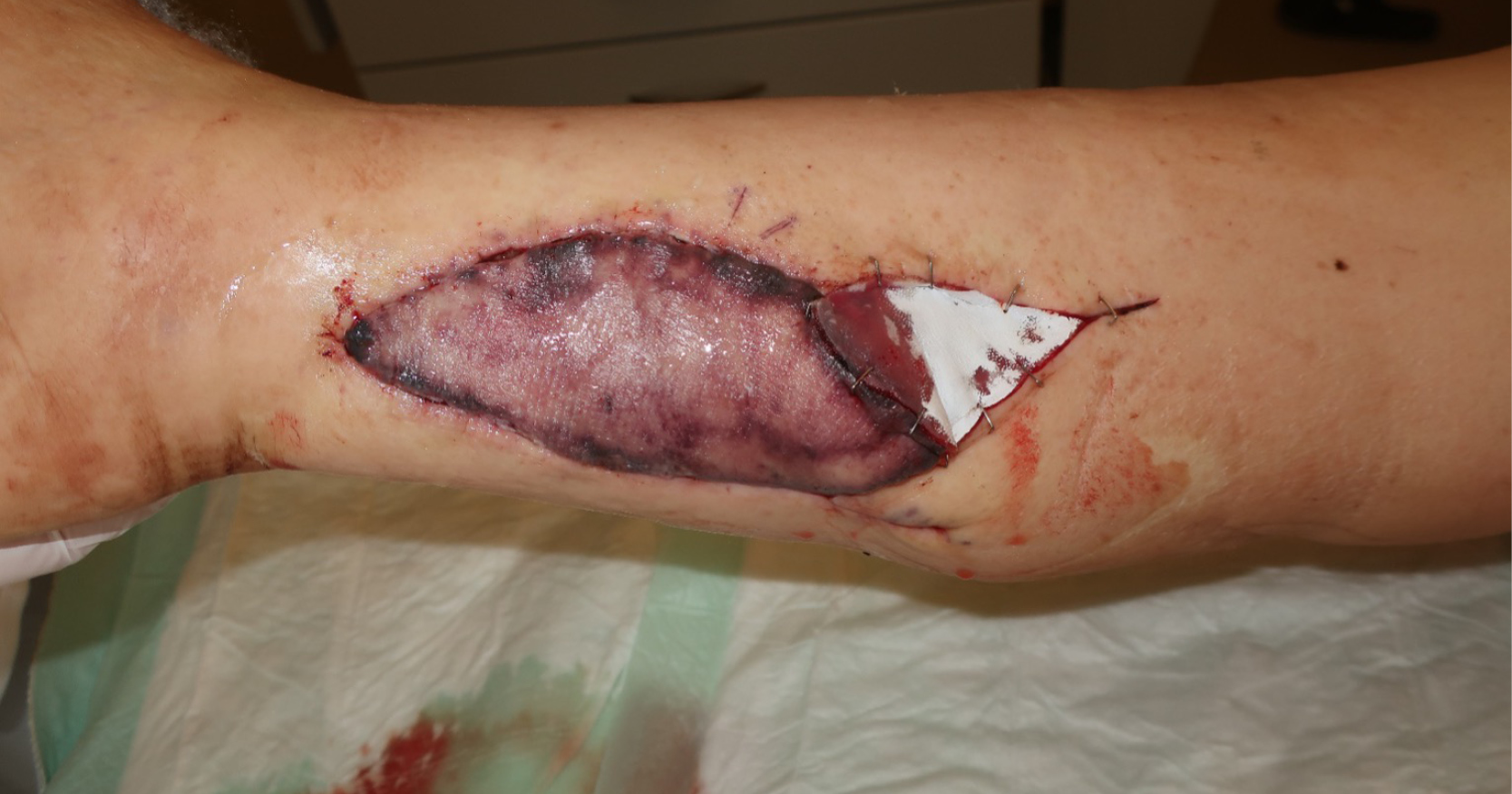
A newborn baby was operated on immediately after birth for intestinal atresia and after 30 days for intestinal occlusion. The latter postoperative period was totally uneventful and the baby was receiving saline solution at 15ml/hour through a vascular access on his left wrist, protected by a splint. The pump connected to the venous line had no alarm device to detect any possible problem. During the night, the catheter displaced and fluid extravasation occurred. The next day, the left upper limb was swollen up to the left side of the neck, the hand and fingers were cold and no radial pulse was detectable. Blisters were present on the forearm up to the elbow and on the palm of the hand [Figure 1]. Degloving of the fingers was also observed. The baby was immediately taken to the operating theatre and multiple escharotomies were performed. After a few minutes local oedema slowly decreased. The limb was debrided and covered with a skin substitute from the hand up to the elbow [Figure 2]. No anomalies were detected on doppler echo scan. On day 8 postoperative, the skin substitute was removed and the skin appeared to be healed. Escharotomy wounds were treated with advanced nanocrystalline silver dressings until complete healing by secondary intention was achieved on day 21 [Figure 3].
Case 2
A 3-month-old baby was operated on for plagiocephaly (a flat spot on one side of the head or the whole back of the head). A venous line was placed on his left ankle connected to a pump for pressurised infusion of fluids. The device had no alarm system too. On undraping the patient, after a 7-hour neurosurgical procedure, the left leg appeared to be tensely swollen and cyanotic, mild clinical sign of skin damage due to vascular impairment. Multiple escharotomies were performed and surgical wounds were covered with a skin substitute for 10 days [Figure 4]. Advanced nanocrystalline silver dressings were subsequently used to achieve complete healing [Figure 5].
Results
Emergency surgical decompression of the limbs affected by ACS in both cases was successful in dramatically reducing the severity of its clinical outcome. In cases 1 and 2, the use of a skin substitute was necessary to treat the skin damage due to vascular impairment, to achieve fast and robust re-epithelisation of the limbs. Advanced dressings were extremely useful in achieving complete healing by secondary intention of escharotomy incisions, avoiding local infections and allowing for an easy management at home by caregivers. Scars were treated with silicon sheets and custom-made pressure garments and both children are still followed up by physiotherapists, even if no functional impairment of involved joints is present [Figure 3 and 5].
Discussion
Extravasation occurs in up to 11% of young children and accounts for a significant number of complications of peripheral intravenous use in children (Rodger and Hammerschlag, 2018).
Early diagnosis of ACS of the limbs caused by fluid extravasation and its possible emergency surgical decompression represents the therapeutic ‘golden standard’ of this serious condition.
However, recognition of ACS in children can be challenging because the constellation of signs and symptoms typical of adults have a late presentation in this population (Livingston et al, 2017). On the other hand, Noonan and McCarthy proposed the three As (increasing anxiety, agitation, analgesic requirement) to be more appropriate for diagnosing ACS in children (Noonan and McCarthy, 2010).
Careful surveillance of the vascular accesses is therefore mandatory, especially when the venous lines are connected to pressurised infusion pumps of fluids without alarm devices to detect the possible extravasation into the surrounding tissues. Nurses have a important role in the prevention of this dangerous complication. All staff must consider the risks associated with ACS, being able both to early identify signs and symptoms, and being aware of any appropriate medical and nursing procedures to be performed (Harvey, 2001; Hadaway, 2007). That means prevention, detection and treatment.
As in adults, the only effective treatment of ACS in children is decompressive escharotomy and fasciotomy and time is a crucial factor. Few papers about ACS due to fluid extravasation in children are available in literature and the timing of surgical treatment is debated.
Although the functional outcomes after paediatric ACS are good, some papers report that in the absence of fractures, especially in the postoperative and vascular groups, only 56% of patients achieve a full recovery due to delayed diagnosis (Livingston et al, 2017).
On the other hand, a recent systematic review did not reveal a statistically significant difference in time from injury to fasciotomy between patients who achieved full functional recovery and those who did not (Lin and Samora, 2020). The authors stated that paediatric patients generally achieve good outcomes even when presenting in delayed fashion and undergoing fasciotomies for at least 24 hours and recommend considering decompressive fasciotomy in children even if there is prolonged time from injury to diagnosis (Lin and Samora, 2020).
In the cases presented here, a surgical decompression was performed after a delayed diagnosis of ACS, skin substitutes and advanced dressing were used to manage skin lesions and a good outcome was achieved.
Conclusions
To conclude, early diagnosis and treatment of ACS due to fluid extravasation is advocated to achieve good clinical and aesthetical outcomes.
In both cases we presented, the identification of the ACS was immediately followed by emergency surgical decompression of the affected limbs. Skin lesions were managed with skin substitutes and advanced dressings, in order to avoid the possible onset of local infections, promote the physiological healing process and favour an aesthetically and functionally valid scar result.