Head and neck lymphoedema occurs as a consequence of the failure of damaged lymphatics to sufficiently drain lymph from the soft tissues of the head and neck (Földi et al, 2006). Damage to the lymphatic nodes and vessels in the head and neck from cancer treatment may result in lymph stasis and dermal backflow or reverse filling of lymphatic capillaries (Rasmussen et al, 2017). Head and neck lymphoedema can cause chronic oedema, tissue fibrosis, increased susceptibility to infection and body image distress leading to altered social functioning for the individual (Marshall et al, 2010; Ridner et al, 2016; Melissant et al, 2020). Head and neck lymphoedema not only affects the surface of the head and neck, but also internal tissues, potentially leading to dysphagia and airway stenosis (Ridner et al, 2016).
The prevalence of lymphoedema following head and neck cancer is reported to be as high as 90% (Deng et al, 2012; Queija et al, 2019; Jeans et al, 2021). However, head and neck lymphoedema is underdiagnosed so many people do not receive treatment Stubblefield and Weycker 2023). A lack of knowledge about postoperative anatomical changes associated with head and neck lymphoedema and the lack of suitable imaging for examination may hinder accurate lymphoedema diagnosis.
Better understanding of normal and altered lymphatic anatomy after cancer treatment is critical for diagnosis and effective conservative management of head and neck lymphoedema. The anatomic complexity of the lymphatic system in an individual’s head and neck region requires detailed examination to identify variations from common patterns, particularly after surgery and radiotherapy (Sharma et al, 2008).
Computed tomography (CT) and magnetic resonance imaging (MRI) are commonly used for postoperative monitoring of cancer recurrence and may potentially be used to provide imaging information about increases in soft tissue space of the affected region. However, these techniques do not have the capability to visualise the lymphatic vessels; furthermore, anatomic variability in tissue size may show the presence of soft tissue damage after surgical and radiotherapy interventions rather than lymphoedema (Aulino et al, 2018).
Lymphoscintigraphy is used to identify lymph structures for sentinel node detection in head and neck cancer (Skanjeti et al, 2021). It has long been considered the gold standard imaging technique for diagnosis of limb lymphoedema (Polomska et al, 2019). However, it lacks the spatial resolution to detect individual lymphatic vessels or pathways (Zaleska and Olszewski, 2018). Therefore, imaging the lymphatic system is not feasible with conventional imaging modalities.
Indocyanine green (ICG) lymphography is an alternate imaging technique for demonstrating the lymphatic system. It allows real-time visualisation of functional superficial lymphatic drainage pathways (Groenlund et al, 2017). This imaging technique has been used for identification of sentinel nodes in head and neck cancers because it causes less disturbance of signal around the injection site than lymphoscintigraphy and can be used in real-time during surgery (Skanjeti et al, 2021; Thammineedi et al, 2021).
ICG lymphography is used with increasing frequency in limb lymphoedema for lymphoedema diagnosis, selection of surgical procedures, lymphatic mapping to inform lymphoedema surgery, imaging guidance of personalised manual lymphatic drainage (Lopera et al, 2017; Koelmeyer et al, 2021). The diagnostic criterion for limb lymphoedema identified with ICG lymphography is the presence of a pathological and specific imaging sign called ‘dermal backflow’ (Yamamoto et al, 2011b; Chang et al, 2013). Dermal backflow is a phenomena of reflux of lymph from the lymph collecting vessel in the subcutaneous tissue to the lymph pre-collectors in the dermal layer (Suami et al, 2012; Yamamoto, 2016).
ICG lymphography in the head and neck region is an emerging application with some reports of its use to identify sites for sentinel lymph node biopsy in head and neck cancers, in surgical planning, and for the identification of lymph leakage after neck dissection (Kamiya et al, 2009; Chan et al, 2018; Zeng et al, 2019).
Previous works have explored the potential of ICG lymphography for head and neck lymphoedema diagnosis (Yamamoto et al, 2011a; Maus et al, 2012) and pneumatic compression evaluation (Gutierrez et al, 2019), with these studies concluding that the dermal backflow sign could be used to diagnose and inform some conservative therapy planning for head and neck lymphoedema, akin to its application in extremity lymphoedema.
Despite the increasing reports of ICG lymphography applications in limb lymphoedema, there is limited published research to guide potential applications specific to head and neck lymphoedema. Therefore, this exploratory study aimed to describe the use of ICG lymphography in the head and neck and explore whether it could identify lymphatic structures and drainage patterns in a case series of participants with head and neck lymphoedema.
Materials and methods
Design
This study was conducted at a single centre, the Princess Alexandra Hospital, Brisbane, Australia. A single examination with ICG lymphography was conducted. A consecutive case series design was employed to demonstrate the procedure used for head and neck ICG lymphography (Dekkers et al, 2012). Ethical clearance was received from the hospital and university ethics committees HREC/2019/QMS/47451 .
Setting and participants
The study was conducted from March 2019 to March 2020. All patients referred to the hospital lymphoedema service during this period were offered inclusion in the study if they:
- Were over 18 years of age.
- Had a diagnosis of oral, nasopharyngeal, oropharyngeal, laryngeal or hypopharyngeal cancer treated with chemoradiation, surgical interventions with postoperative radiation, or definitive radiation with curative intent.
- Had a rating of 1a or higher on the MD Anderson Cancer Centre Head and Neck Lymphoedema Rating Scale (MDACC Rating Scale), indicating soft visible oedema with no pitting, that was reversible (Smith and Lewin, 2010).
Participants were excluded from the study if they had cancer recurrence; had undergone radiotherapy and/or surgery to the neck, oral, pharyngeal, or laryngeal anatomic structures prior to the current episode of care; had comorbidities associated with head and neck oedema; or, if they were allergic to indocyanine green, sodium iodide or iodine; or, reported having an over-active thyroid, benign tumours of the thyroid, or kidney failure. All participants provided informed written consent prior to participation.
ICG lymphography procedure
The participant was seen in a standard medical treatment room containing an adjustable examination bed and the ICG lymphography equipment. A cryogenic device (CoolSense; Coolsense Medical Ltd., Tel Aviv, Israel) was applied to the participant’s skin for 5 seconds to cool the surface of the skin to reduce injection pain (Suami et al, 2018). Approximately 0.1–0.15 ml of an ICG dye solution (Verdye 25 mg reconstituted with 5 ml 0.9% sodium chloride) was injected intradermally. This off-label use of Verdye was performed under approval from the Therapeutic Goods Administration, Australia’s regulatory authority for therapeutic goods. The ICG dye solution was injected at three routine sites: two temporal sites (targeting upper jugular lymph nodes); and, one nasion site (targeting submandibular lymph nodes). One additional optional injection site in the chin beneath the lower lip (targeting submental lymph nodes) was used if required (Figure 1). Two out of six participants required this additional injection to identify drainage to all lymphatic territories including the submental lymph nodes. These sites were selected to represent the anatomical territories of expected lymphatic flow.
A near-infrared camera system (Photodynamic Eye Neo II; Hamamatsu Photonics KK., Japan) was used to observe the movement of dye fluorescence through the superficial lymphatics. Initial dye uptake was observed with minimal interference (0–5 minutes). Thereafter, the area surrounding the injection was pressurised to facilitate dye uptake into the lymphatics. The procedure was performed with continuous observation and intermittent recording of dye movement. It was continued until no new changes were identified on the image. The procedure took 30–60 minutes.
ICG lymphography guided manual lymphatic drainage (MLD) was conducted from the site of injection to the direction of observed lymph flow by an occupational therapist who had advanced training in lymphoedema management. Any identified lymphatic vessels and demarcation of dermal backflow patterns visualised were marked on the skin. At the completion of the procedure, the markings were transferred onto an illustrative diagram by the therapist present for the procedure. A series of still photographs of the ICG lymphography image skin markings were also captured.
Outcome measures
The participant’s external head and neck lymphoedema was assessed and described by the occupational therapist present during the procedure using the MDACC Rating Scale (Smith and Lewin, 2010). This four-point ordinal scale describes lymphoedema stages as: 0 (no visible oedema but patient reports heaviness); 1a (soft visible oedema, no pitting, reversible); 1b (soft pitting oedema, reversible); 2 (firm pitting oedema, not reversible, no tissue changes); and, 3 (irreversible, tissue changes).
ICG lymphography images were evaluated using a bespoke audit tool to document key imaging features including lymphatic movement features and MLD observations. Audit tool features included: direction of flow; appearance of vessels (linear or area of dermal backflow), speed of flow (rapid (0–5 minutes), intermediate (15–30 minutes), delayed (>30 minutes) or did not occur); location of the identified nodal regions; barriers to lymph flow; and pressure of massage required to move lCG dye.
Imaging data analysis
In keeping with the small convenience sample size and exploratory nature of the study, participant demographics were reported descriptively. The ICG lymphography procedure photographs were assessed against the audit tool described above. Assessment occurred collaboratively during the procedure by two members of the research team who had extensive clinical lymphoedema experience over 12 years each (MT, AM) and extensive experience reporting on vascular images for 14 years (AM). To minimise bias, a third member of the research team with 18 years’ clinical lymphoedema experience (AP) subsequently audited images and where discrepancies existed, procedure recordings were reviewed for further analysis until consensus was reached.
Results
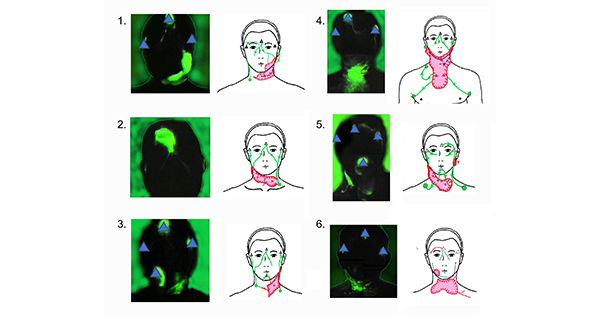
All patients approached consented to participation. Six participants (four men; two women) with a median age of 57.8 years (range 46–71 years) underwent the ICG lymphography procedure for head and neck lymphoedema (Table 1). Lymphatic movement observed for each participant is represented in Figure 2 and further described in Table 1.
Linear lymphatic vessels were initially identified from all injection sites. In unilateral neck dissection cases (Cases 1 and 3), linear lymphatic vessels were traced from the injection site to the expected lymph node regions in the non-surgical side. However, in the neck dissection side, the lymphatic vessels were interrupted by dermal backflow and the dermal backflow extended to the opposite side and connected to the lymph node regions in the non-surgical neck.
In the bilateral neck dissection cases (Cases 2, 4 and 6), dermal backflow was identified in the bilateral neck. The drainage regions were identified in either the remaining lymph nodes in the neck region or in the axillary region in Case 4. In Case 5, no surgery, only radiation treatment was received. This participant demonstrated dermal backflow in the irradiated neck, which suggested that head and neck lymphoedema may be present despite the absence of precipitating surgical interventions.
Barriers to the extension of dermal backflow were observed and in most of the cases the surgical scar and neck skin fold interrupted the altered lymph flow. MLD observations demonstrated that all participants required very firm pressure to move the ICG dye through the areas of dermal backflow. ICG dye flow moved with only light pressure in areas with linear lymphatic vessels.
Discussion
This study aimed to explore the potential clinical use of ICG lymphography in conservative management of head and neck lymphoedema. Potential uses of the clinical information obtained were hypothesised to include diagnosis and development of individualised MLD pathways for conservative management. The images obtained showed that lymphatic vessels of the head and neck (including the presence of dermal backflow) were visible in all participants using the procedure described. The procedure could be performed in the outpatient setting with individual lymphatic drainage visualised in real time.
The findings for Cases 1 and 3 suggested that the unaffected side of the neck represented a normal control image and dermal backflow was not identified at this side. Lymph collecting vessels were identified from each injection site in these cases and their courses were identical with anatomical atlases (Rouvière, 1981, Pan et al, 2008, Mascagni, 1787), and connected to the corresponding regional lymph nodal basins. On the other hand, dermal backflow was consistently identified in the operated side and dermal backflow extended to the contralateral side or to the axillary node as in Case 4. The presence of dermal backflow has already been reported as representing lymphoedema diagnosis in extremity lymphoedema (Yamamoto et al, 2011b, 2011c) and our findings suggested that it was reasonable to expand this clinical application to head and neck lymphoedema. This may facilitate earlier diagnosis of head and neck lymphoedema enabling earlier conservative treatment as is the standard of care in upper limb lymphoedema (Koelmeyer et al, 2019; Ridner et al, 2022)
The procedure enabled the impact of MLD on lymph flow to be observed. Requirements for MLD were similar to findings identified for extremity lymphoedema (Koelmeyer et al, 2021). In areas of dermal backflow, firm and slow hand manoeuvre was essential to move ICG dye through superficial lymphatics, whereas ICG dye was seen to move easily with lighter faster massage in areas without dermal backflow.
There was variability in the location of lymphatic flow pathways and drainage points for each participant in our study. This is consistent with the individual alteration of the lymphatic drainage pathway to the residual lymph nodal regions identified with ICG lymphography after cancer treatment in other areas of the body (Heydon-White et al, 2020; Koelmeyer et al, 2021). This finding is also supported by a previous study of head and neck lymphatic pathways after cancer treatment which used lymphoscintigraphy and SPECT/CT, to identify unexpected drainage pathways in 30% of participants (n=43) different from the original condition (den Toom et al, 2019). Given the rates of unexpected drainage in the head and neck, ICG lymphography is considered as a useful tool to design bespoke treatment programs for individuals.
This paper presents findings from a small case series. Due to the small number of participants and study design, the findings cannot be generalised to the broader population and require replication in a larger sample. Despite this, the results demonstrate that ICG lymphography has potential as a diagnostic imaging tool. Particular benefits of the procedure include the absence of a radioactive agent, it can be performed in an outpatient setting and it is able to identify dermal backflow. Further research may consider the standard use of the four nominated injection sites as standard for all participants. Expected outcomes of ICG lymphography for head and neck lymphoedema include imaging for lymphoedema diagnosis in the outpatient clinic setting and ICG-guided personalised conservative management. These preliminary results support plans to progress to larger scale research in the head and neck region, as has already been investigated in other body regions.
Conclusion
This preliminary study demonstrated ICG lymphography can be used to identify lymphatic structures and drainage patterns in the head and neck region. ICG lymphography has potential for use in lymphoedema diagnosis and conservative treatment planning of MLD for head and neck lymphoedema.