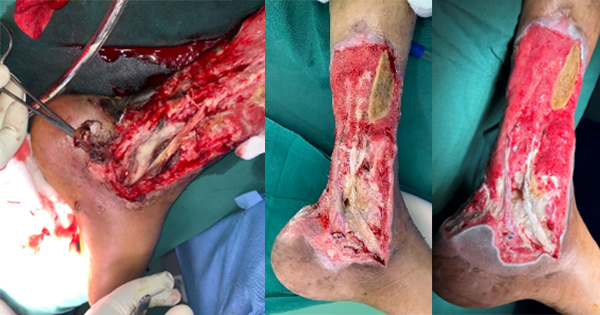
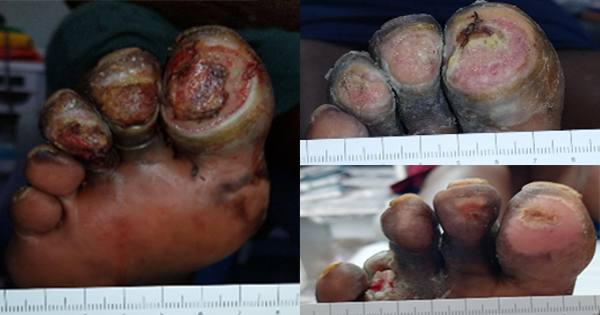
The skin is the largest organ in the body and serves numerous functions, including acting as a physical, chemical and microbiological barrier to protect the host as the first line of defence against external pathogens (Nguyen and Soulika, 2019; Chambers and Vukmanovic; Stejic, 2020). Any break in this protective barrier allows the invasion of a wound by proliferating microorganisms to a level that may invoke a response in the host and cause sustained inflammation, thereby delaying healing (Tom et al, 2019; Yang et al, 2024). The World Health Organization (WHO, 2013) suggests that open injuries have the potential for serious bacterial wound infections, which may lead to long-term disabilities, chronic wounds or bone infections and death.
Wound infection
A wound can be considered infected when the presence and subsequent proliferation of microorganisms leads to a local response in an individual (Sandoz, 2022). It is the result of a multiplication of pathogenic microorganisms that evoke a prolonged and excessive inflammatory response, leading to delayed healing, an increased risk of hospital admission, and implications for patients, including increased pain and reduced quality of life, as well as a significant burden on healthcare systems (Rondas, 2016; Falcone et al, 2021; Sandoz, 2022).
The risk of wound infection increases with the degree of contamination, immunosuppressive status and exposure to a dirty environment, and it has been estimated that about 50% of wounds contaminated with bacteria become clinically infected (Tom et al, 2019; International Wound Infection Institute (IWII), 2022). Wounds can be colonised by a wide range of organisms, many of which have natural resistance to various antibiotics (Matsuura et al, 2013; Tom et al, 2019).
Skin microbiota includes diverse microorganisms, i.e. bacteria, fungi, viruses, and yeasts, and plays a significant role in the protection of skin tissue and the maintenance of haemostasis. However, while commensal and pathogenic bacteria are in equilibrium in normal skin, their imbalance in the wound area can cause delayed or impaired cutaneous wound healing (Ersanli et al, 2023).
Anaerobic bacteria have been reported to propagate wound chronicity and biofilm production and have been identified as a major contributor to wound bioburden (Ersanli et al, 2023). As aerobic bacteria create biofilms on the exterior of deep wounds, anaerobic bacteria invade the interior (Hussain et al, 2016).
The classic signs and symptoms of local infection include:
- Redness or erythema
- Warmth
- Swelling or induration
- Pain or tenderness
- Pus or purulent secretions.
These signs and symptoms are the first indications for the diagnosis of local wound infection (Lipsky et al, 2016; Ward and Holloway, 2019). Other signs may include friable or discoloured granulation tissue, pocketing, undermining of the ulcer rim, foul odour, delayed healing or deterioration of wound progression (deepening or extending), while systemic signs or symptoms such as fever, chills and elevated inflammatory markers, are often associated with spreading wound infection (Lipsky et al, 2016).
Moreover, microbiological investigations based on biopsies performed at the level of the depth of the infection diagnosed (e.g. bone, abscess, soft tissue) provide information on the identification of the organisms and their sensitivities to antibiotics (IWII, 2022).
Antimicrobials in wound care
Antimicrobials are therapeutic substances used to prevent or treat infections; these include antimicrobial dressings, which play an important role in the management of soft tissue infections (Roberts, 2016; Di Martino, 2022).
The use of antiseptics to prevent and treat wound infection is increasingly being studied, mainly because of the rapid spread of antimicrobial resistance and the rising emergence of multidrug-resistant microorganisms (Nair et al, 2023). While the most commonly used topical agents in wounds are targeted against planktonic cells, some have been adopted for use in managing chronic wounds complicated by biofilm (Schwarzer et al, 2019).
Clinicians must evaluate various factors concerning the wound when selecting an appropriate antimicrobial dressing, with crucial considerations, including wound size, depth, presence of necrotic tissue, foreign material, the level and type of exudate, and the risk and signs and symptoms of local infection (Yousefian et al, 2023).
Silver dressings in wound management
Silver has been used as an antimicrobial since ancient times, as early as 1850 BCE Egypt, where it was directly applied to wounds to improve healing (Dissemond et al, 2017). Silver, in its elemental form, is inert and only becomes bactericidal in the wound when it is ionised upon contact with an aqueous solution such as wound exudate (Fletcher et al, 2021). Silver ions are bactericidal by binding to the bacterial cell wall, thus preventing nutrients and oxygen from entering the cell. The silver ions are then transported through the cell membrane and into the cell, where they prevent the cell from producing further energy and alter the sequence of the DNA (Percival and McCarty, 2015).
While the efficacy of silver dressings has been challenged by the Vulcan Trial (Michaels et al, 2009), the study’s findings were considered potentially misleading, as the silver dressings were not used in line with clinical recommendations and, therefore, could not be expected to provide clinically relevant information on efficacy (Fletcher et al, 2021). Conversely, other clinical trials concluded that the use of silver dressings improves healing time and can lead to overall cost savings (Lazareth, 2008; Jemec et al, 2014). Moreover, a meta-analysis published in 2017 (Dissemond et al) shows that “the evidence base for silver in wound management is significantly better than perceived in the current scientific debate. Thus, if used selectively and for a limited period of time, silver not only has antimicrobial effects but is also characterised by an improvement in quality of life and good cost-effectiveness”.
Technology Lipido-Colloid Mesh with Silver Sulphate (TLC-Ag)
Non-adherent and non-occlusive mesh dressings with Technology Lipido-Colloid with Silver (TLC-Ag, UrgoTul Ag® – Laboratoires Urgo, France) are indicated for wounds that are presenting with signs and symptoms of local infection or are at risk of infection (White et al, 2011). In vitro analyses showed that, from day one and throughout the duration of the study (seven days), a reduction in the number of colony-forming units for all the bacterial strains studied (including methicillin-resistant S. aureus, P. aeruginosa, S. pyogenes and C. albicans) was noted, making it possible to conclude that the TLC-Ag dressing demonstrates antibacterial efficacy on the microorganisms tested (White et al, 2011).
TLC-Ag has been demonstrated to promote wound healing through its support of a moist wound healing environment, which also allows for atraumatic removal, providing antimicrobial efficacy (Meaume et al, 2004; Lazareth et al, 2008). A sequential randomised control trial was conducted by Lazareth et al (2008) with patients with venous leg ulcers presenting with at least three out of five clinical signs. Patients were randomly treated either with the TLC-Ag dressing or with a neutral TLC dressing for 4 weeks. Then, subjects in both groups were managed for an additional 4 weeks with TLC dressings (without silver), totalling 8 weeks. The intention to treat population included 99 subjects. This randomised control trial demonstrated that a 4-week treatment with the TLC-Ag dressing resulted in both a reduction of clinical signs of infection and a sustained increase in the closure rate of venous leg ulcers presenting inflammatory signs suggesting a high bacterial load, compared to a neutral dressing.
In a prospective, multicentre, observational study, 728 patients with wounds of various aetiologies and wound infection statuses were treated with the TLC-Ag dressings in 39 centres for a mean duration of 26±19 day (Lützkendorf et al, 2022). At the initial visit, it was established that the majority of patients (60.4%) had a wound infection, while the remaining cohort presented with first clinical signs of a local wound infection (25.1%) or were at risk of wound infection (13.2%). Throughout the study period, all the parameters of wound infection continuously decreased, resulting at the final visit in a reduction of 78.9% in the prevalence of local wound infections and 72.0% in the clinical signs of wound infection. Concurrently, in terms of the healing process, 92.1% of the wounds healed or improved, 3.2% remained unchanged and 1.7% worsened (data missing for 3.0%), and an improvement of the periwound skin was reported in 65.7% of the patients.
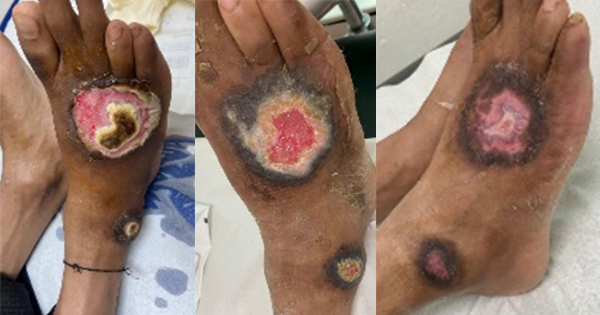
The TLC-Ag dressing was also previously evaluated in India, but mainly on burn wounds (Shanker, 2019; Uppal et al, 2020). The authors of this case series wanted to document the TLC-Ag dressing more widely across different aetiologies to increase the evidence base for the dressing’s performance in the management of patients with wounds in India.
Conclusion
Wound infection is one of the most common and potentially devastating complications of the wound healing process (Celik et al, 2024). When inadequately managed, wound infection hinders wound healing, prolongs the inflammatory process, and may lead to secondary complications. In some cases, amputation, resulting in a cycle of pain, anxiety, and reduced quality of life for the patient, as well as increased treatment costs (Kim, 2019; Celik et al, 2024). Moreover, Indian studies on the epidemiology of chronic wounds estimate the prevalence at a rate of 4.5 per 1,000 population, and it is suggested that untreated or inadequately treated acute traumatic wounds are a frequent cause of these hard to heal wounds (Monika et al, 2022). Implementation of effective strategies to prevent, diagnose, and manage wound infection is important in reducing the burden of wounds, as well as mortality and morbidity rates (IWII, 2022).
It should be noted that climate and humidity in India play a significant role in wound healing and may require adapted approaches to wound care (Morgan-Jones et al, 2024).
The IWII 2022 guidelines recommend early recognition and management of factors that may predispose a wound to infection and, furthermore, emphasise that the implementation of evidence-based care is fundamental for wound infection prevention and management. They advise that effective management should include optimising the individual host response, reducing local microbial burden, and promoting a positive environment for wound healing.
The TLC-Ag non-adherent wound dressing has been demonstrated as an effective antimicrobial that can restart the healing process in wounds presenting clinical signs of infection. The dressing’s silver ions’ ability to control a high bacterial load and provide anti-microbial properties appears to promote a favourable microenvironment and foster a sustained decrease in wound surface area in wounds presenting a high risk of infection (Lazareth et al, 2008).
Although the TLC-Ag dressing has been evaluated in India, this was primarily in burn wounds (Shankar, 2019; Uppal, 2020). The clinician authors saw a need to evaluate the dressing in different aetiologies. The patients noted and appreciated the pain-free dressing changes, and one could observe the positive healing outcomes with good aesthetic and re-pigmentation in most of the wounds.
These cases represent only a small cohort of patients, but the results are in line with those of other published trials and provide a positive platform to substantiate the inclusion of the TLC-Ag dressing as part of their standard of care for wounds presenting with signs and symptoms of local infection or at risk of infection.