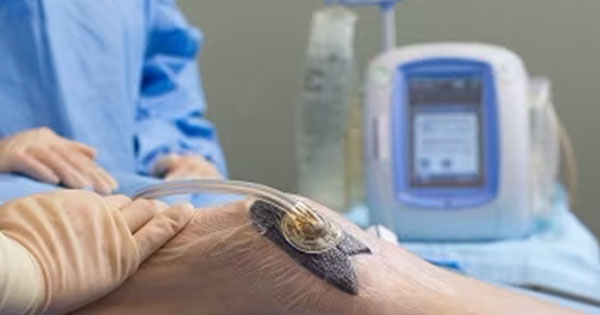
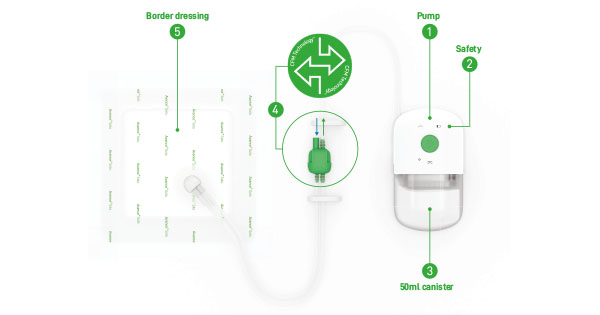
Application of negative pressure wound therapy (NPWT) to an injured site is widely used to improve the healing of wounds, as described in Vikatmaa et al (2008). As several references have shown (Vikatmaa et al, 2008; Hasan et al, 2015), NPWT aids in the healing process by reducing oedema and encouraging new tissue formation, or granulation, at the wound site. Although the benefits of NPWT are well established, the risk for infection with this therapy remains an area of ongoing concern, as described in Armstrong and Lavery (2005). Some fairly recent studies (Armstrong and Lavery, 2005; Gabriel et al, 2008; 2012; Kim et al, 2013; Anghel et al, 2016) have investigated the impact of antiseptics, antimicrobials and antifungal solutions via wound instillation on both patient and hospital outcomes.
The ideal methods for utilising wound instillation in patient care is also not clear. Guidance published by Kim et al (2013) suggests an appropriate dwell time for wound instillation is between 10 to 20 minutes; however, the optimal dwell time remains unclear and, in the case of antimicrobials, may depend on the pharmacodynamics of the instilled product. Specific recommendations for instillation volume are even less precise, given variations in wound size and quality. It is important to apply enough volume to bathe the surface of the wound, but not so much as to inhibit maintaining a seal with the occlusive dressing. There are presently no guidelines that recommend dwell times or drug concentration of the instillation specific to vancomycin.
Given the lack of literature describing vancomycin wound instillation with NPWT, the following case report seeks to describe the clinical course of one patient receiving NPWT with vancomycin instillation. Secondly, it is our intention to describe the process improvement that was implemented to ensure patient safety due to the limited guidance for providers and pharmacists monitoring of vancomycin wound instillation.
Case presentation
A 57-year-old Caucasian female weighing 128kg arrived in our intensive care unit (ICU) in respiratory failure secondary to suspected aspiration and sepsis, following recent cholangitis with a modified early warning score of five. She was intubated prior to arrival at an outside long-term care hospital (LTCH). A propofol infusion was initiated at 40 mcg/kg/minute shortly after arrival to the ICU and titrated to Richmond Agitation Sedation Scale of -1. As a result of the decline in blood pressure associated with the propofol infusion, a phenylephrine infusion was initiated at 0.8mcg/kg/minute and titrated to a mean arterial pressure of >65mmHg. Initial arterial blood gas measurement revealed a pH of 7.22, a partial pressure of carbon dioxide of 84 mmHg, and a bicarbonate of 34mEq/L. The patient’s past medical history included hypertension, hyperlipidemia, type 2 diabetes mellitus, depression, gastroesophageal reflux disease and morbid obesity.
Chest computed tomography (CT) revealed infiltrates suggestive of aspiration pneumonia with empyema, for which she was empirically started on cefepime 2,000mg intravenously (IV) every 8 hours and vancomycin 1,750mg IV every 12 hours. Five days later, a CT scan of the abdomen showed significant ascites and interventional radiology drainage revealed purulence. On hospital day (HD) 5, an exploratory laparotomy was performed for abscess drainage and enterolysis. Cultures of the pleural fluid grew methicillin-resistant Staphylococcus aureus (MRSA) sensitive to vancomycin and Stenotrophomonas maltophilia sensitive to levofloxacin on HD six. Cefepime was discontinued and levofloxacin was initiated at a dose of 750mg IV every 24 hours.
On HD 10, an exploratory laparotomy was performed, revealing an intra-abdominal abscess measuring 8.7cm in length and 5.5cm in width. Vancomycin 1 mg/mL wound instillation was ordered for the intra-abdominal wound and administered as 100mL with a dwell time of 10 minutes every 3 hours. Throughout the course of the patient’s stay, IV vancomycin was ordered concomitantly with vancomycin wound instillation on HD 10, 19, 26, and 30 (Table 1). On HD 20, the intra-abdominal wound size decreased, allowing for the reduction of the vancomycin instillation volume to 50mL. On HD 21, the vancomycin instillation volume could be decreased to 25mL. Duration and frequency remained the same on these days. Intravenous vancomycin doses were adjusted per facility treatment protocol, with a goal trough of 15–20 mcg/mL. Supra-therapeutic trough levels were observed for the first 6 days of concomitant vancomycin IV and instillation therapy. On HD 32, the patient developed KDIGO Stage 2 AKI in the setting of significant third spacing, which resolved after administration of a sodium chloride 0.9% 500mL bolus followed by dextrose 5% plus sodium chloride 0.45% at 100mL/hour. The patient progressed to the point of discharge on HD 54 to a LTCH without vasopressor support and was able to undergo spontaneous breathing trials during the day. The first 34 HDs are explored in more detail in Table 1.
Case summary
This patient received vancomycin wound instillation with NPWT for an intra-abdominal related wound. Vancomycin 1000 mg was mixed in 1000 mL of sodium chloride 0.9% (1 mg/mL) and initially instilled at 100 mL with a dwell time of 10 to 30 minutes. The vancomycin was instilled in cycles every 3 hours, alternating with a suction of low intermittent 125 mmHg pressure continuously. The patient received IV vancomycin concomitantly on days 10–19 and 26–30. During both treatment timeframes, supra-therapeutic vancomycin troughs were observed. The patient developed an AKI on HD 32 following 5 days of concomitant IV and instilled vancomycin.
In response to concerns for potential systemic absorption of instilled vancomycin following extended therapy, Sanford Medical Center Fargo developed a structured approach for administering and monitoring patients on NPWT with vancomycin instillation. Although there are presently no guidelines directing drug concentration of vancomycin instillation, studies have looked at the toxic effects of vancomycin when exposed to in vitro skeletal cells via local delivery systems. Antoci et al (2007) concluded concentrations of vancomycin less than or equal to 1mg/mL had little impact on cell proliferation and reported concentrations greater than 1mg/mL were associated with impaired osteoblast proliferation. A standardised order panel now allows physicians to prescribe vancomycin 1,000mg in 1,000mL of 0.9% sodium chloride (1mg/mL) for instillation via V.A.C VeraFloTM to prevent topical administration of vancomycin at concentrations greater than 1mg/mL due to concern for local cell toxicity. The order panel sends a consult for pharmacy to monitor random vancomycin levels at the discretion of the pharmacist while the patient is receiving vancomycin instillations. This consult additionally allows pharmacy to discontinue concomitant IV vancomycin pending indication and cultures to prevent duplicate therapy.
Conclusion
Vancomycin wound instillation via V.A.C VeraFlo may be a beneficial approach in treating open wounds. Structuring our approach to vancomycin wound instillation has helped us better establish the management of antimicrobial wound instillation with NPWT at our institution and promote patient safety through close monitoring for renal impairment. Additional studies are required to establish and quantify the efficacy and safety of vancomycin wound instillation with NPWT.